Can the −CF3 Group Act as a Tight, Well-Defined Hydrogen Bond Acceptor? A Clear Crystallographic CF2–F···H–N+ Interaction Says Yes
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
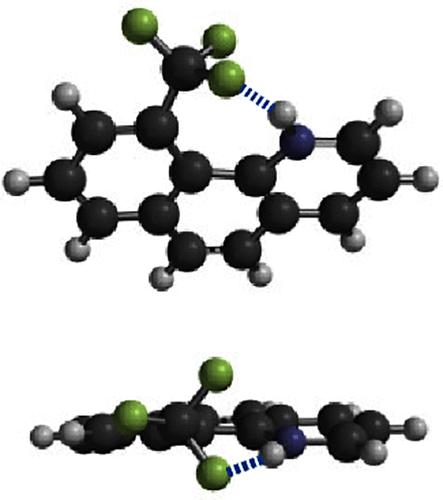
The CF3 group is well noted for being noninteractive with other functional groups. In this Note, we present a highly rigid model system containing a significant hydrogen bonding interaction between a charged N–H donor and a CF3 acceptor that challenges this accepted wisdom. Spectroscopic and single crystal X-ray crystallography data characterize this interaction, consistent with a weak to moderate hydrogen bond that would be difficult to observe in an intermolecular system.
-CF3基团能否充当紧密、明确的氢键受体?清晰的晶体学 CF2-F-H-N+ 相互作用证明了这一点
众所周知,CF3 基团与其他官能团没有相互作用。在本说明中,我们介绍了一个高度刚性的模型体系,该体系包含一个带电的 N-H 给体和一个 CF3 受体之间的重要氢键相互作用,对这一公认的观点提出了挑战。光谱和单晶 X 射线晶体学数据表明,这种相互作用与分子间体系中难以观察到的弱至中等程度的氢键相一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


