Efficacy and Safety of Ensitrelvir for Asymptomatic or Mild COVID-19: An Exploratory Analysis of a Multicenter, Randomized, Phase 2b/3 Clinical Trial
Abstract
Background
This phase 2b/3, randomized, placebo-controlled trial explored the efficacy and evaluated the safety of ensitrelvir. This trial involved individuals with asymptomatic infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and patients with mild symptoms of coronavirus disease 2019 (COVID-19).
Methods
The trial was conducted at 57 medical institutions in Japan, South Korea, and Vietnam (study period: January 6–August 14, 2022). Eligible participants were randomized (1:1:1) to the ensitrelvir 125-mg, ensitrelvir 250-mg, or placebo group, received the allocated intervention orally, and were followed up until Day 28. Participants self-rated the severity of 14 typical COVID-19 symptoms and recorded the data in an electronic diary.
Results
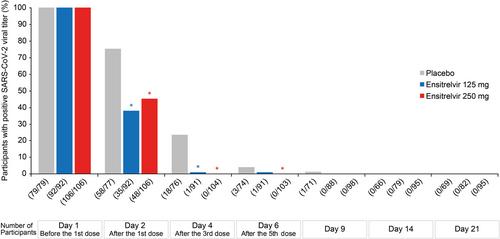
In total, 572 participants (194, 189, and 189 in the ensitrelvir 125-mg, ensitrelvir 250-mg, and placebo groups, respectively) were included in the intention-to-treat population. Ensitrelvir 125-mg group observed a 77% reduction in the risk of developing any of the 14 COVID-19 symptoms or fever and a 29% reduction in the risk of worsening of such symptoms or fever versus placebo (statistically nonsignificant). The viral RNA, viral titer, and time to infectious viral clearance observed a statistically significant decrease versus placebo. Most treatment-related adverse events (TEAEs) were mild to moderate in severity, and the most common TEAE observed across groups was a decrease in high-density lipoprotein.
Conclusions
Our exploratory results suggest a potential reduction in the risk of development or worsening of COVID-19 symptoms with ensitrelvir. Ensitrelvir showed antiviral efficacy and was well tolerated.
Trial Registration: Japan Registry of Clinical Trials identifier: jRCT2031210350.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


