CAM-A-dependent HBV core aggregation induces apoptosis through ANXA1
IF 9.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
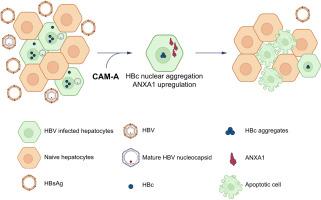
Chronic HBV infection is the leading cause of liver disease and of hepatocellular carcinoma. The improvement of antiviral therapy remains an unmet medical need. Capsid assembly modulators (CAMs) target the HBV core antigen (HBc) and inhibit HBV replication. Although CAM-A compounds are well-known inducers of aberrant viral capsid aggregates, their mechanisms of action in HBV-hepatocyte interactions are poorly understood. Recently, we demonstrated that CAM-A molecules lead to a sustained reduction of HBsAg in the serum of HBV replicating mice and induce HBc aggregation in the nucleus of HBc-expressing cells leading to cell death.
Methods
The mechanism of action by which CAM-A compounds induce cell death was investigated using an HBV infection model, HBc-overexpressing HepG2-NTCP cells, primary human hepatocytes, and HBV replicating HepAD38 cells.
Results
We first confirmed the decrease in HBsAg levels associated with CAM-A treatment and the induction of cell toxicity in HBV-infected differentiated HepaRG cells. Next, we showed that CAM-A-mediated nuclear aggregation of HBc was associated with cell death through the activation of apoptosis. Transcriptomic analysis was used to investigate the mechanism of action driving this phenotype. CAM-A-induced HBc nuclear aggregation led to the upregulation of ANXA1 expression, a documented driver of apoptosis. Finally, silencing of ANXA1 expression delayed cell death and apoptosis in CAM-A-treated cells, confirming its direct involvement in CAM-A-induced cell death.
Conclusions
Our results unravel a previously undiscovered mechanism of action involving CAM-As and open the door to new therapeutic strategies involving CAM to achieve a functional cure in patients with chronic infections.
Impact and implications:
Chronic HBV infection is a global health threat. To date, no treatment achieves viral clearance in chronically infected patients. In this study, we characterized a new mechanism of action of an antiviral molecule targeting the assembly of the viral capsid (CAM). The study demonstrated that a CAM subtype, CAM-A-induced formation of aberrant structures from HBV core protein aggregates in the nucleus leading to cell death by ANXA1-driven apoptosis. Thus, CAM-A treatment may lead to the specific elimination of HBV-infected cells by apoptosis, paving the way to novel therapeutic strategies for viral cure.
CAM-A 依赖性 HBV 核心聚集通过 ANXA1 诱导细胞凋亡
背景& 目的 慢性 HBV 感染是导致肝病和肝细胞癌的主要原因。改善抗病毒治疗仍是一项尚未满足的医疗需求。囊壳组装调节剂(CAMs)靶向 HBV 核心抗原(HBc)并抑制 HBV 复制。虽然 CAM-A 化合物是众所周知的异常病毒荚膜聚集诱导剂,但它们在 HBV-肝细胞相互作用中的作用机制却鲜为人知。最近,我们证实 CAM-A 分子可导致 HBV 复制小鼠血清中的 HBsAg 持续下降,并诱导 HBc 在表达 HBc 的细胞核中聚集,导致细胞死亡。方法使用 HBV 感染模型、HBc 高表达的 HepG2-NTCP 细胞、原代人类肝细胞和 HBV 复制的 HepAD38 细胞研究了 CAM-A 复合物诱导细胞死亡的作用机制。接着,我们发现 CAM-A 介导的 HBc 核聚集与细胞凋亡的激活有关。转录组分析被用来研究驱动这种表型的作用机制。CAM-A 诱导的 HBc 核聚集导致了 ANXA1 表达的上调,而 ANXA1 是细胞凋亡的驱动因子。最后,沉默 ANXA1 的表达可延缓 CAM-A 处理细胞的细胞死亡和凋亡,这证实了 ANXA1 直接参与了 CAM-A 诱导的细胞死亡。结论:我们的研究结果揭示了一种以前未被发现的 CAM-As 作用机制,并为涉及 CAM 以实现慢性感染患者功能性治愈的新治疗策略打开了大门。迄今为止,还没有一种治疗方法能清除慢性感染患者体内的病毒。在这项研究中,我们揭示了一种针对病毒外壳组装(CAM)的抗病毒分子的新作用机制。研究表明,一种 CAM 亚型(CAM-A-induced formation of aberrant structures from HBV core protein aggregates in the nucleus)可导致细胞因 ANXA1 驱动的细胞凋亡而死亡。因此,CAM-A 治疗可通过细胞凋亡特异性地消除受 HBV 感染的细胞,为新的病毒治疗策略铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


