Production of alkyl levulinates as a versatile precursor by phosphomolybdate-impregnated g-C3N4 catalysts
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-01-25
DOI:10.1016/j.jiec.2024.06.018
引用次数: 0
Abstract
Alkyl levulinates are a class of compounds that have diverse applications and show great promise as environmentally friendly fuel additives. These compounds are renewable, have a high energy density, and can easily be used with existing infrastructure. Therefore, they can play a significant role in contributing to the ongoing endeavors toward more sustainable and efficient energy solutions. In this study, alkyl levulinates (ALs) as fuel additives were synthesized using phosphomolybdate g-C3N4 (PMox/g-C3N4) as an efficient catalyst for the esterification reaction of levulinic acid (LA) with three diverse alcohols, namely ethanol, 1-butanol, and 1-hexanol. An experimental investigation assessed the impact of several key factors, such as temperature, the volumetric proportion of LA to alcohol, reaction time, catalyst loading, and catalyst amount, on the chemical process under study. For ethyl levulinate (EL) a 76 % yield was obtained at 130 °C, 8 h reaction time, LA: ethanol 1:12, and 20 mg catalyst. Also, a > 99 % yield was obtained for the butyl levulinate (BL) production at 130 °C, 8 h reaction time, LA: alcohol proportion of 1:12, and 10 mg catalyst. Hexyl levulinate (HL) was achieved with 71 % yield under the optimal condition of 230 °C, 8 h reaction time, LA: hexanol 1:20, and 10 mg catalyst.
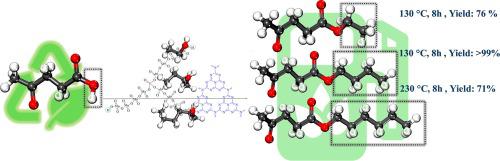

利用磷钼酸盐浸渍 g-C3N4 催化剂生产作为多功能前体的左旋烷基酚
乙酰丙酸烷基酯是一类应用广泛的化合物,是一种很有前景的环保燃料添加剂。这些化合物是可再生的,具有高能量密度,并且可以很容易地与现有的基础设施一起使用。因此,他们可以在不断努力寻求更可持续和高效的能源解决方案方面发挥重要作用。本研究以磷酸钼酸酯g-C3N4 (PMox/g-C3N4)为催化剂,催化乙酰丙酸(LA)与乙醇、1-丁醇和1-己醇的酯化反应,合成了乙酰丙酸烷基酯(al)作为燃料添加剂。一项实验研究评估了几个关键因素,如温度、LA与醇的体积比、反应时间、催化剂负载和催化剂用量,对所研究的化学过程的影响。对于乙酰丙酸乙酯(EL),在130°C, 8 h反应时间,LA:乙醇1:12,20mg催化剂下,收率为76%。还有,a >;在130℃、反应时间8 h、LA:醇比1:12、催化剂10 mg条件下,乙酰丙酸丁酯(BL)的收率为99%。在反应温度230℃,反应时间8 h, LA:己醇1:20,催化剂10 mg的条件下,乙酰丙酸己酯(HL)的收率为71%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


