Self-pressurizing nanoscale capsule catalysts for CO2 electroreduction to acetate or propanol
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The selective one-step CO2 electroreduction reaction (CO2RR) to acetate and propanol has garnered intense interest. Here we report the design of self-pressurizing nanoscale capsule catalysts for the CO2RR. A high-pressure CO intermediate environment is created around copper catalysts by a permselective enclosure. Microkinetic modelling, 13CO2/12CO co-feed experiments and in situ Raman spectroscopy confirm that a unique CO–CO2 coupling path is involved, which is only initiated at high CO intermediate pressure. This pathway benefits acetate production due to the kinetic and energetic advantages of COCO2*. The acetate Faradaic efficiency is 38.5 ± 2.2% (8 times higher than that achieved without enclosure) and the acetate partial current density is 328 ± 19 mA cm−2, which surpasses the performance of previous CO2RR catalysts. In situ investigation indicates that the CO pressure inside the nanoscale capsule catalysts can reach 8 ± 3 bar. Furthermore, self-pressurizing nanoscale capsule catalysts with a CuI-derived core can reduce CO2 to propanol with a Faradaic efficiency of 25.7 ± 1.2% and a conversion rate of 155 ± 3 mA cm−2. CO2 electroreduction to multicarbon products is desirable but challenging. Now, self-pressurizing nanoscale capsule catalysts are synthesized. The self-pressurising capsules harness high-pressure CO environments for selective acetate or propanol production via a CO–CO2 coupling pathway.
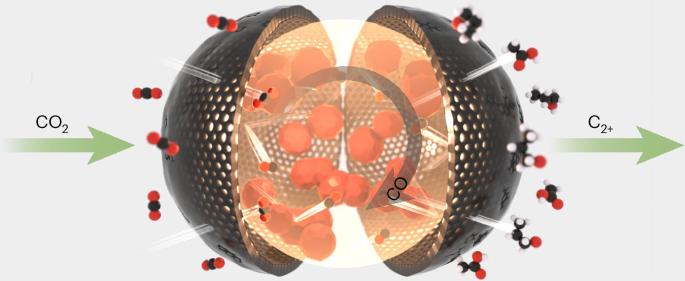
用于将二氧化碳电还原为醋酸或丙醇的自加压纳米级胶囊催化剂
一步法选择性 CO2 电还原反应(CO2RR)生成醋酸酯和丙醇引起了人们的浓厚兴趣。在此,我们报告了用于 CO2RR 的自加压纳米级胶囊催化剂的设计。铜催化剂周围通过包覆选择性形成了高压 CO 中间环境。微动力学建模、13CO2/12CO 共馈实验和原位拉曼光谱证实,其中涉及一种独特的 CO-CO2 耦合途径,该途径仅在高 CO 中间压力下启动。由于 COCO2* 在动力学和能量方面的优势,这一途径有利于醋酸盐的生产。醋酸酯法拉第效率为 38.5 ± 2.2%(是无封闭情况下的 8 倍),醋酸酯部分电流密度为 328 ± 19 mA cm-2,超过了以往 CO2RR 催化剂的性能。原位研究表明,纳米胶囊催化剂内部的 CO 压力可达 8 ± 3 巴。此外,以 CuI 为核心的自加压纳米级胶囊催化剂可将二氧化碳还原为丙醇,其法拉第效率为 25.7 ± 1.2%,转化率为 155 ± 3 mA cm-2。将 CO2 电还原为多碳产品是一种理想但具有挑战性的方法。现在,我们合成了自加压纳米级胶囊催化剂。自加压胶囊利用高压 CO 环境,通过 CO-CO2 偶联途径选择性地生产醋酸或丙醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
文献相关原料
公司名称
产品信息
阿拉丁
Cu(CH3COO)2·H2O
阿拉丁
N2H4·H2O
阿拉丁
1,3,5-trimethylbenzene
阿拉丁
ethylene glycol
阿拉丁
KI
阿拉丁
Na2S·9H2O
阿拉丁
KOH

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


