C(sp3)–C(sp3) bond formation through nitrogen deletion of secondary amines using O-diphenylphosphinylhydroxylamine
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
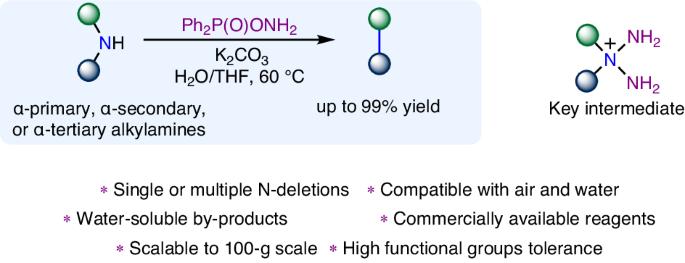
The nitrogen deletion of secondary amines has emerged as an effective strategy for direct molecular skeletal editing and carbon–carbon bond formation. However, current methods are often limited to acyclic bis(α-primary) amines and cyclic amines, which possess two stabilizing elements at the α-position of amine. Here we report the use of O-diphenylphosphinylhydroxylamine as a reagent for nitrogen deletion of secondary amines to form C(sp3)–C(sp3) bonds. This method overcomes substrate requirements of other methods and tolerates a range of secondary amine substrates. The process can be readily applied to multiple nitrogen deletion processes, is tolerant of both air and water, forms water-soluble byproducts and can be readily scaled to a hundred-gram scale. The versatility of the method is further showcased through the direct editing of natural products, pharmaceutical compounds, N-coordinated ligands, a three-dimensional amine cage and the synthesis of several bioactive compounds. Methods for nitrogen deletion of secondary amines are often limited by substrate structure requirements. Now the use of O-diphenylphosphinylhydroxylamine for nitrogen deletion of a range of secondary amines is reported. The developed process is tolerant of both air and water and can be scaled easily.
使用 O-二苯基膦酰羟胺通过仲胺脱氮形成 C(sp3)-C(sp3)键
仲胺的氮缺失已成为一种直接进行分子骨架编辑和碳-碳键形成的有效策略。然而,目前的方法往往局限于无环双(α-原位)胺和环胺,因为它们在胺α位拥有两个稳定元素。在此,我们报告了使用 O-二苯基膦酰羟胺作为试剂对仲胺进行脱氮以形成 C(sp3)-C(sp3)键的方法。该方法克服了其他方法对底物的要求,并可容忍一系列仲胺底物。该工艺可随时应用于多种脱氮工艺,对空气和水都有耐受性,可形成水溶性副产物,并可随时扩展到百克级规模。通过直接编辑天然产物、药物化合物、N 配位配体、三维胺笼以及合成多种生物活性化合物,进一步展示了该方法的多功能性。仲胺的脱氮方法通常受到底物结构要求的限制。现在报告了使用 O-二苯基膦酰羟胺对一系列仲胺进行脱氮的方法。所开发的工艺对空气和水都有很好的耐受性,而且易于扩展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


