Crystallization-Induced Diastereomeric Transformation of Chiral Ozanimod Key Intermediate Using Homogeneous Ir-Based Racemization Catalyst
IF 3.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Crystallization-induced diastereomeric transformation of a solid solution salt composed of a chiral primary amine, 4-cyano-1-aminoindane, and di-p-toluoyl-l-tartaric acid was achieved using (pentamethylcyclopentadienyl)iridium(III) diiodide dimer as a racemization catalyst. The enrichment continued until the composition of the solid phase was in equilibrium with that of the liquid phase at 0% diastereomeric excess.
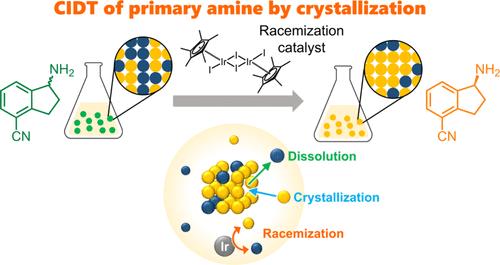
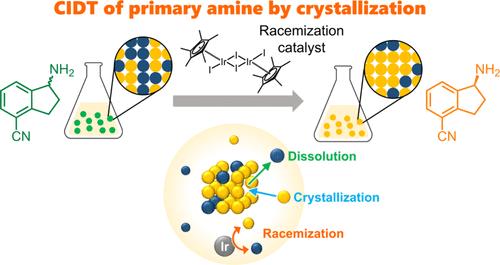
利用均相铱基消旋化催化剂实现手性奥扎莫德关键中间体的非对映异构结晶转化
使用(五甲基环戊二烯基)二碘化铱(III)二聚体作为消旋化催化剂,实现了由手性伯胺、4-氰基-1-氨基茚满和二对甲苯甲酰基-l-酒石酸组成的固溶体盐的结晶诱导非对映转化。富集过程一直持续到固相成分与液相成分达到平衡,非对映过量为 0%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Crystal Growth & Design
化学-材料科学:综合
CiteScore
6.30
自引率
10.50%
发文量
650
审稿时长
1.9 months
期刊介绍:
The aim of Crystal Growth & Design is to stimulate crossfertilization of knowledge among scientists and engineers working in the fields of crystal growth, crystal engineering, and the industrial application of crystalline materials.
Crystal Growth & Design publishes theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. Synergistic approaches originating from different disciplines and technologies and integrating the fields of crystal growth, crystal engineering, intermolecular interactions, and industrial application are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


