Horner–Wadsworth–Emmons olefination of proteins and glycoproteins
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
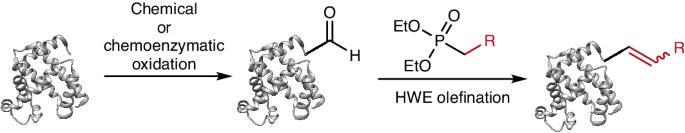
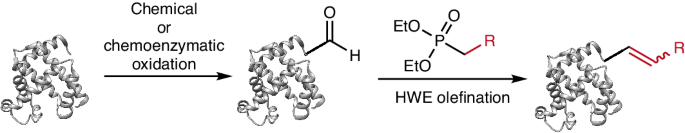
Chemo-selective modifications of proteins are fundamental to the advancement of biological and pharmaceutical sciences. The search for biocompatible chemical reactions has prompted us to investigate Horner–Wadsworth–Emmons (HWE) olefinations, iconic reactions in organic synthesis that would give rise to new selective protein olefinations. Our choice of HWE olefinations was inspired by the growing number of methods for generating aldehydes as transient reactive groups in proteins and the potential for mild and simple reaction conditions. Here we show that HWE olefination reactions on aldehydes, produced by both chemical and enzymatic methods, are compatible with physiological conditions and highly selective in small and large proteins, including therapeutic antibodies and stable recombinant proteins exemplified by green fluorescent protein. Reaction kinetics can be fine-tuned over orders of magnitude both by judicious use of substituents and pH regulation. The electrophilic nature of the HWE olefination products can be tuned to allow for subsequent nucleophilic additions, including thiol- and phospha-Michael additions. Our results demonstrate that HWE olefination of aldehydes in proteins provides efficient and selective bioconjugation chemistries that are orthogonal to existing methods. Aldehyde-bearing proteins are shown to be suitable substrates for Horner–Wadsworth–Emmons reactions. Applying this process to proteins and glycoproteins enables site-specific bioconjugation with tunable reaction kinetics.
蛋白质和糖蛋白的霍纳-沃兹沃斯-埃蒙斯油化作用
蛋白质的化学选择性修饰是生物和制药科学发展的基础。寻找生物相容性化学反应促使我们研究霍纳-沃兹沃斯-埃蒙斯(HWE)油化反应,这是有机合成中的标志性反应,可产生新的选择性蛋白质油化反应。我们之所以选择 HWE 烯化反应,是因为有越来越多的方法可以在蛋白质中生成作为瞬时反应基团的醛,而且反应条件温和简单。在这里,我们展示了通过化学和酶法产生的醛的 HWE 烯化反应,这种反应符合生理条件,对小型和大型蛋白质(包括治疗性抗体和以绿色荧光蛋白为例的稳定重组蛋白)具有高度选择性。通过合理使用取代基和调节 pH 值,可以对反应动力学进行数量级的微调。HWE 烯化产物的亲电性可以进行调整,以便进行后续的亲核加成,包括硫醇加成和磷酸-迈克尔加成。我们的研究结果表明,蛋白质中醛类的 HWE 烯化反应提供了与现有方法截然不同的高效、选择性生物连接化学方法。结果表明,含醛蛋白质是霍纳-沃兹沃斯-艾蒙斯反应的合适底物。将这一过程应用于蛋白质和糖蛋白可实现特定位点的生物共轭,并可调节反应动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


