Cryo-EM structures of Smc5/6 in multiple states reveal its assembly and functional mechanisms
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
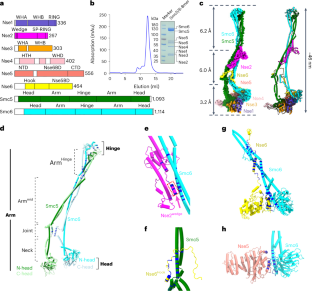
Smc5/6 is a member of the eukaryotic structural maintenance of chromosomes (SMC) family of complexes with important roles in genome maintenance and viral restriction. However, limited structural understanding of Smc5/6 hinders the elucidation of its diverse functions. Here, we report cryo-EM structures of the budding yeast Smc5/6 complex in eight-subunit, six-subunit and five-subunit states. Structural maps throughout the entire length of these complexes reveal modularity and key elements in complex assembly. We show that the non-SMC element (Nse)2 subunit supports the overall shape of the complex and uses a wedge motif to aid the stability and function of the complex. The Nse6 subunit features a flexible hook region for attachment to the Smc5 and Smc6 arm regions, contributing to the DNA repair roles of the complex. Our results also suggest a structural basis for the opposite effects of the Nse1–3–4 and Nse5–6 subcomplexes in regulating Smc5/6 ATPase activity. Collectively, our integrated structural and functional data provide a framework for understanding Smc5/6 assembly and function. Cryo-EM structures covering full-length yeast Smc5/6 in three states and the accompanying mutagenesis data reveal multiple new structural and functional features of this unique SMC complex.

多种状态下 Smc5/6 的冷冻电镜结构揭示了其组装和功能机制
Smc5/6是真核生物染色体结构维护(SMC)复合物家族的成员,在基因组维护和病毒限制中发挥着重要作用。然而,对Smc5/6结构的有限了解阻碍了对其多种功能的阐释。在此,我们报告了芽殖酵母 Smc5/6 复合物在八亚基、六亚基和五亚基状态下的冷冻电镜结构。这些复合体全长的结构图揭示了复合体组装的模块化和关键元素。我们发现,非 SMC 元素(Nse)2 亚基支持复合体的整体形状,并使用楔形图案来帮助复合体的稳定性和功能。Nse6 亚基具有一个灵活的钩区,可连接到 Smc5 和 Smc6 的臂区,有助于发挥该复合体的 DNA 修复作用。我们的研究结果还表明,Nse1-3-4 和 Nse5-6 亚复合物在调节 Smc5/6 ATPase 活性方面具有相反作用的结构基础。总之,我们的综合结构和功能数据为了解 Smc5/6 的组装和功能提供了一个框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


