An incentive-based text-messaging intervention to reduce maternal alcohol use during pregnancy and lactation in South Africa (MaRISA study): Findings from a single-arm pilot study
Abstract
Background
South Africa has the highest rate of fetal alcohol spectrum disorders (FASD) globally. As with alcohol use during pregnancy, alcohol consumption while breastfeeding adversely impacts infant development. We pilot tested an incentive-based text-messaging intervention to reduce alcohol use during pregnancy and lactation in South Africa.
Methods
A single-arm pilot trial was conducted over 3 months in healthcare facilities in Cape Town, South Africa. Pregnant and breastfeeding participants tested positive for recent alcohol use by urinalysis. The three-month intervention had two components, contingency management of alcohol abstinence confirmed by urinalysis twice weekly and weekly health-related text messaging from an evidence-based brief intervention. We collected twice weekly urine samples for measurement of ethyl glucuronide (EtG), an alcohol biomarker, and measures of self-reported alcohol and drug use, violence exposure, and mental health at six weeks and three months post-enrollment.
Results
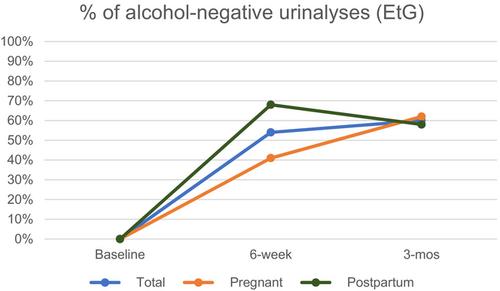
Sixty participants were enrolled, of whom 31 were pregnant and 29 lactating. The number of days with four or more drinks in the past month decreased from 9 days at baseline, on average, to 1–3 days (p-value range: 0.144–0.010) at follow-up timepoints. There were statistically significant increases in the proportions of participants with alcohol-negative urine tests (p < 0.001). The percentages of participants breastfeeding while using alcohol decreased from baseline to the end of 3 months in the overall sample and among those enrolled postpartum, though these were not significant (p-value range: 0.255–0.147). Maternal depression scores also decreased among participants enrolled postpartum (p = 0.054). Emotional abuse by the main partner, but neither physical nor sexual abuse, significantly decreased at both follow-ups in the overall sample (p = 0.032) and among participants enrolled while pregnant (p = 0.015).
Conclusions
This study is among the first to pilot test an incentive-based text-messaging intervention for maternal alcohol use and other outcomes such as depression and violence exposure. Further testing is warranted in a well-powered, randomized controlled trial.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


