Efficacy and safety of once daily oral administration of sodium-glucose cotransporter-2 inhibitor velagliflozin compared with twice daily insulin injection in diabetic cats
Abstract
Background
Options for treatment of diabetes mellitus in cats are limited to insulin injections and monitoring for hypoglycemia.
Hypothesis
Once daily sodium-glucose cotransporter-2 inhibitor velagliflozin PO is noninferior to insulin injections.
Animals
Client-owned diabetic cats (127 safety; 116 efficacy assessment).
Methods
Prospective, randomized (1 mg/kg velagliflozin), positive controlled (titrated Caninsulin), open label, noninferiority field trial, comparing number of cats with treatment success in ≥1 clinical variable and ≥1 glycemic variable (margin Δ: 15%) on Day 45; secondary endpoints included glycemic and clinical assessments during 91 days.
Results
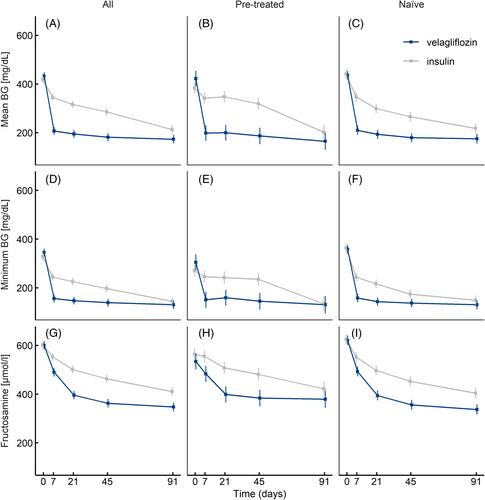
On Day 45, 29/54 (54%) velagliflozin-treated cats and 26/62 (42%) Caninsulin-treated cats showed treatment success, demonstrating noninferiority (difference −11.8%; upper 1-sided 97.5% confidence interval, −∞ to 6.3%). By Day 91, quality of life (QoL), polyuria, and polydipsia had improved in 81%, 54% and 61% (velagliflozin); on blood glucose (BG) curves, mean BG was <252 mg/dL in 42/54 (78%; velagliflozin) and 37/62 (60%; Caninsulin); minimum BG was <162 mg/dL in 41/54 (76%; velagliflozin) and 41/62 (66%; Caninsulin); serum fructosamine was <450 μmol/L in 41/54 (76%; velagliflozin) and 38/62 (61%; Caninsulin). Velagliflozin's most frequent adverse events were loose feces/diarrhea (n = 23/61, 38%), positive urine culture (n = 19/61, 31%), and nonclinical hypoglycemia (BG <63 mg/dL; n = 8/61, 13%); Caninsulin's: clinical and nonclinical hypoglycemia (n = 35/66, 53%), positive urine culture (n = 18/66, 27%), and loose feces/diarrhea (n = 10/66, 15%). Diabetic ketoacidosis occurred in 4/61 (7%; velagliflozin) and 0/66 (Caninsulin).
Conclusions and Clinical Importance
Once daily oral administration of velagliflozin was noninferior to insulin injections, showed good QoL and glycemia without clinical hypoglycemia.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


