Cobalt Catalyzed α-Hydroxylation of Arylacetic Acid Equivalents with Dioxygen
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A cobalt catalyst, under oxidative conditions, facilitates the single electron transfer process in N-pyridyl arylacetamides to form α-carbon-centered radicals that readily react with molecular oxygen, giving access to mandelic acid derivatives. In contrast to the known benzylic hydroxylation approaches, this approach enables chemo- and regioselective hydroxylation at a benzylic position adjacent to (N-pyridyl)amides. Mild conditions, broad scope, excellent selectivity, and wide synthetic practicality set up the merit of the reaction.
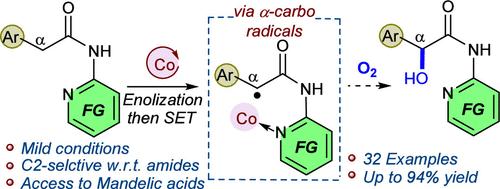
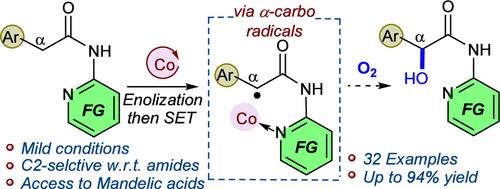
钴催化芳基乙酸当量与二氧的α-羟化反应。
在氧化条件下,钴催化剂可促进 N-吡啶基芳基乙酰胺中的单电子转移过程,形成易于与分子氧反应的 α 碳中心自由基,从而获得扁桃酸衍生物。与已知的苄基羟化方法不同,这种方法可以在邻近(N-吡啶基)酰胺的苄基位置进行化学和区域选择性羟化。温和的条件、广泛的范围、出色的选择性和广泛的合成实用性奠定了该反应的优点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


