Faulty Gap Filling in Nucleotide Excision Repair Leads to Double-Strand Break Formation in Senescent Cells
IF 5.7
2区 医学
Q1 DERMATOLOGY
引用次数: 0
Abstract
The change of repair efficiency of UV-induced pyrimidine dimers due to aging was examined in replicatively senesced fibroblasts. The fibroblasts with repeated passages showed the characteristics of cellular senescence, including irreversible cell cycle arrest, elevated β-galactosidase activity, and senescence-associated secretory phenotype. The incision efficiency of oligonucleotide containing UV lesions was similar regardless of cell doubling levels, but the gap filling process was impaired in replicatively senescent cells. The releases of xeroderma pigmentosum group G, proliferating cell nuclear antigen, and replication protein A from damaged sites were delayed, which might have disturbed the DNA polymerase progression. The persistent single-stranded DNA was likely converted to double-strand breaks, leading to ataxia telangiectasia-mutated phosphorylation and 53BP1 foci formation. Phosphorylated histone H2AX (γ-H2AX) induction mainly occurred in G1 phase in senescent cells, not in S phase such as in normal cells, indicating that replication stress–independent double-strand breaks might be formed. MRE11 having nuclease activity accumulated to damaged sites at early time point after UV irradiation but not released in senescent cells. The pharmacological studies using specific inhibitors for the nuclease activity suggested that MRE11 contributed to the enlargement of single-stranded DNA gap, facilitating the double-strand break formation.
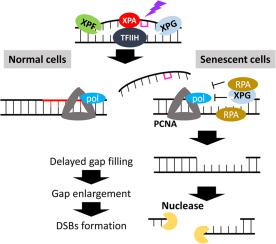
核苷酸切除修复中的缺陷填补导致衰老细胞中双链断裂的形成。
在复制衰老的成纤维细胞中研究了衰老导致的紫外线诱导嘧啶二聚体修复效率的变化。重复传代的成纤维细胞表现出细胞衰老的特征,包括不可逆的细胞周期停滞、β-半乳糖苷酶活性升高和衰老相关的分泌表型。无论细胞倍增水平如何,含有紫外线病变的寡核苷酸的切割效率相似,但在复制衰老的细胞中,间隙填充过程受损。XPG、PCNA和RPA从受损部位释放的时间推迟,这可能干扰了DNA聚合酶的进程。持续存在的单链 DNA(ssDNA)很可能转化为双链断裂(DSB),导致 ATM 磷酸化和 53BP1 病灶的形成。衰老细胞的γ-H2AX诱导主要发生在G1期,而不是像正常细胞那样发生在S期,这表明可能形成了与复制胁迫无关的DSB。具有核酸酶活性的 Mre11 在紫外线照射后的早期积累到受损位点,但在衰老细胞中没有释放出来。使用特异性核酸酶活性抑制剂进行的药理学研究表明,Mre11有助于扩大ssDNA间隙,促进DSB的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.70
自引率
4.60%
发文量
1610
审稿时长
2 months
期刊介绍:
Journal of Investigative Dermatology (JID) publishes reports describing original research on all aspects of cutaneous biology and skin disease. Topics include biochemistry, biophysics, carcinogenesis, cell regulation, clinical research, development, embryology, epidemiology and other population-based research, extracellular matrix, genetics, immunology, melanocyte biology, microbiology, molecular and cell biology, pathology, percutaneous absorption, pharmacology, photobiology, physiology, skin structure, and wound healing

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


