The identification of effective tumor-suppressing neoantigens using a tumor-reactive TIL TCR-pMHC ternary complex
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Neoantigens are ideal targets for cancer immunotherapy because they are expressed de novo in tumor tissue but not in healthy tissue and are therefore recognized as foreign by the immune system. Advances in next-generation sequencing and bioinformatics technologies have enabled the quick identification and prediction of tumor-specific neoantigens; however, only a small fraction of predicted neoantigens are immunogenic. To improve the predictability of immunogenic neoantigens, we developed the in silico neoantigen prediction workflows VACINUSpMHC and VACINUSTCR: VACINUSpMHC incorporates physical binding between peptides and MHCs (pMHCs), and VACINUSTCR integrates T cell reactivity to the pMHC complex through deep learning-based pairing with T cell receptors (TCRs) of putative tumor-reactive CD8 tumor-infiltrating lymphocytes (TILs). We then validated our neoantigen prediction workflows both in vitro and in vivo in patients with hepatocellular carcinoma (HCC) and in a B16F10 mouse melanoma model. The predictive abilities of VACINUSpMHC and VACINUSTCR were confirmed in a validation cohort of 8 patients with HCC. Of a total of 118 neoantigen candidates predicted by VACINUSpMHC, 48 peptides were ultimately selected using VACINUSTCR. In vitro validation revealed that among the 48 predicted neoantigen candidates, 13 peptides were immunogenic. Assessment of the antitumor efficacy of the candidate neoepitopes using a VACINUSTCR in vivo mouse model suggested that vaccination with the predicted neoepitopes induced neoantigen-specific T cell responses and enabled the trafficking of neoantigen-specific CD8 + T cell clones into the tumor tissue, leading to tumor suppression. This study showed that the prediction of immunogenic neoantigens can be improved by integrating a tumor-reactive TIL TCR-pMHC ternary complex. Cancer scientists have created a new way to identify and choose neoantigens (new proteins on cancer cells) for use in cancer vaccines. The research, led by Woong-Yang Park, included 33 patients with different cancer types. The scientists used a mix of computer-based prediction techniques and lab-based tests to find the most effective neoantigens. The results showed that the new technique, named VACINUS, was better at predicting effective neoantigens than older methods. The scientists concluded that the VACINUS technique could improve the immune response of predicted neoantigens, making them better at activating an immune reaction against cancer cells. This could result in the creation of more effective cancer vaccines in the future. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
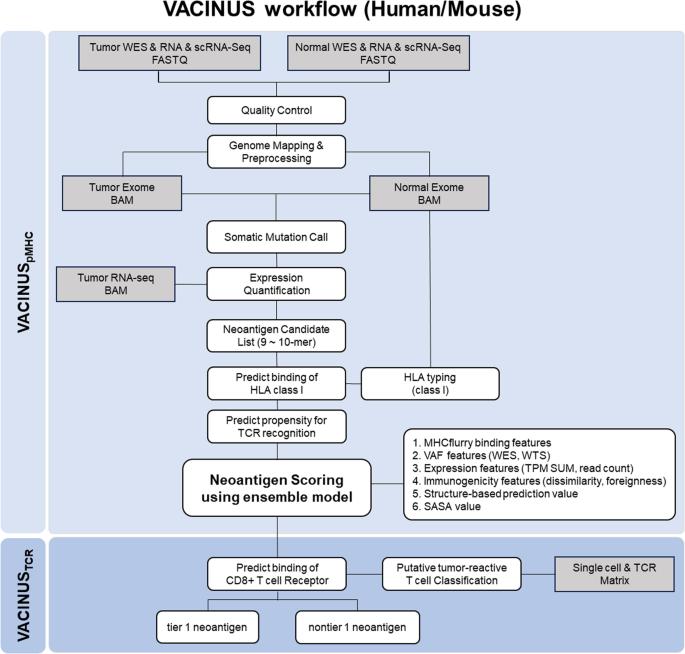
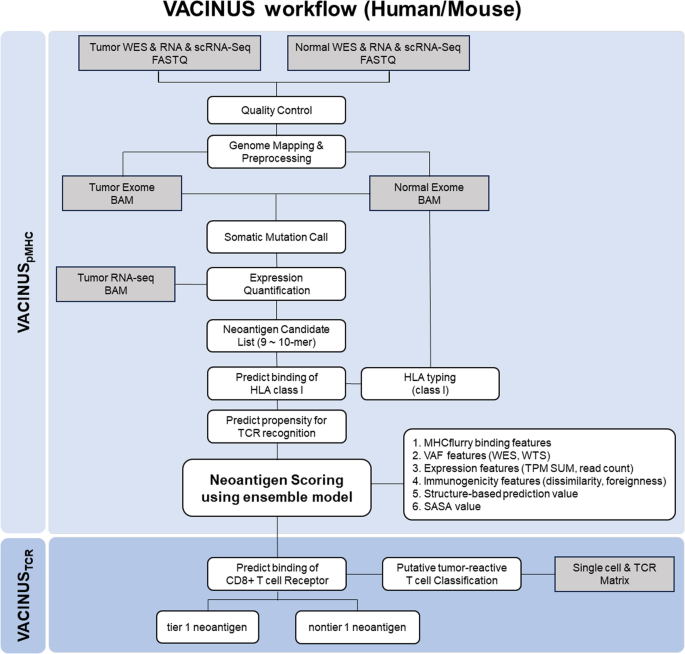
利用肿瘤反应性 TIL TCR-pMHC 三元复合物鉴定有效的肿瘤抑制新抗原。
新抗原是癌症免疫疗法的理想靶点,因为它们在肿瘤组织中从头表达,而在健康组织中不表达,因此被免疫系统识别为外来物。下一代测序和生物信息学技术的进步使得肿瘤特异性新抗原的快速鉴定和预测成为可能;然而,只有一小部分预测的新抗原具有免疫原性。为了提高免疫原性新抗原的可预测性,我们开发了硅学新抗原预测工作流 VACINUSpMHC 和 VACINUSTCR:VACINUSpMHC 结合了肽与 MHC(pMHC)之间的物理结合,而 VACINUSTCR 则通过基于深度学习的配对与假定的肿瘤反应性 CD8 肿瘤浸润淋巴细胞(TIL)的 T 细胞受体(TCR)结合,整合了 T 细胞对 pMHC 复合物的反应性。然后,我们在肝细胞癌(HCC)患者和 B16F10 小鼠黑色素瘤模型中对新抗原预测工作流程进行了体外和体内验证。VACINUSpMHC 和 VACINUSTCR 的预测能力在 8 例 HCC 患者的验证队列中得到了证实。在 VACINUSpMHC 预测的 118 个候选新抗原中,VACINUSTCR 最终筛选出 48 个肽段。体外验证显示,在 48 个预测的候选新抗原中,13 个肽具有免疫原性。使用 VACINUSTCR 体内小鼠模型评估候选新表位的抗肿瘤效果表明,接种预测的新表位可诱导新抗原特异性 T 细胞反应,并使新抗原特异性 CD8 + T 细胞克隆进入肿瘤组织,从而抑制肿瘤。这项研究表明,通过整合肿瘤反应性TIL TCR-pMHC三元复合物,可以改善免疫原性新抗原的预测。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


