Diverse Magnetic Chains in Inorganic Compounds
IF 14
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In both inorganic and metal–organic compounds, transition metals surrounded by ligands form regular or distorted polyhedra, which can be either isolated or interconnected. Distortion of the polyhedron can be caused by the degeneracy in the population of atomic or molecular orbitals, which can be removed by the cooperative Jahn–Teller effect. This effect is often accompanied by the formation of low-dimensional magnetic structures, of which we will consider only chain, or quasi-one-dimensional, magnetic compounds variety. Magnetic chains are formed when transition metal polyhedra bond through a vertex, edge, or face. Moreover, the magnetic entities can be coupled through various nonmagnetic units like NO3, SiO4, PnO3 or PnO4, ChO3 or ChO4, where Pn is the pnictide and Ch is the chalcogen. In most cases, the local environment of the transition metal is represented by oxygen and/or halogens. The prevailing number of chain systems is based on 3d transition metals, albeit 4d and 5d systems attract more and more attention. Mixed 3d–4f single chain magnets became popular objects in metal–organic chemistry.
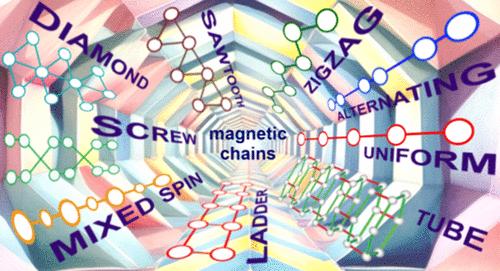
无机化合物中的多种磁链
在无机化合物和金属有机化合物中,配体包围的过渡金属形成规则或扭曲的多面体,这些多面体可以是孤立的,也可以是相互连接的。多面体的扭曲可能是由原子或分子轨道群的退行性引起的,这种退行性可以通过合作的扬-泰勒效应消除。这种效应通常伴随着低维磁性结构的形成,我们将只考虑其中的磁链或准一维磁性化合物种类。当过渡金属多面体通过顶点、边或面结合时,就会形成磁链。此外,磁性实体还可以通过各种非磁性单元耦合,如 NO3、SiO4、PnO3 或 PnO4、ChO3 或 ChO4,其中 Pn 为锑化物,Ch 为钙化物。在大多数情况下,过渡金属的局部环境由氧和/或卤素表示。尽管 4d 和 5d 系统吸引了越来越多的关注,但以 3d 过渡金属为基础的链式系统数量居多。3d-4f 混合单链磁体已成为金属有机化学的热门研究对象。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


