Mechanistic insights into G-protein coupling with an agonist-bound G-protein-coupled receptor
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
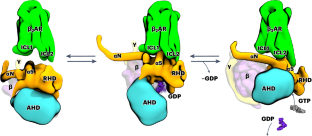
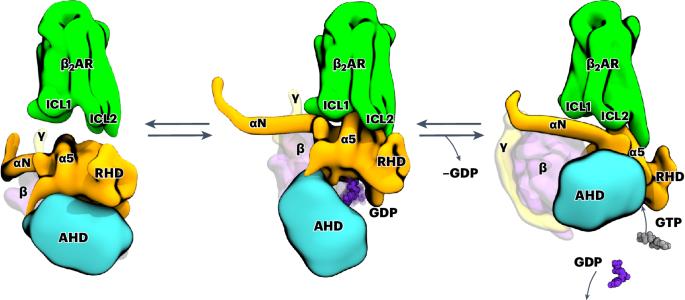
G-protein-coupled receptors (GPCRs) activate heterotrimeric G proteins by promoting guanine nucleotide exchange. Here, we investigate the coupling of G proteins with GPCRs and describe the events that ultimately lead to the ejection of GDP from its binding pocket in the Gα subunit, the rate-limiting step during G-protein activation. Using molecular dynamics simulations, we investigate the temporal progression of structural rearrangements of GDP-bound Gs protein (Gs·GDP; hereafter GsGDP) upon coupling to the β2-adrenergic receptor (β2AR) in atomic detail. The binding of GsGDP to the β2AR is followed by long-range allosteric effects that significantly reduce the energy needed for GDP release: the opening of α1-αF helices, the displacement of the αG helix and the opening of the α-helical domain. Signal propagation to the Gs occurs through an extended receptor interface, including a lysine-rich motif at the intracellular end of a kinked transmembrane helix 6, which was confirmed by site-directed mutagenesis and functional assays. From this β2AR–GsGDP intermediate, Gs undergoes an in-plane rotation along the receptor axis to approach the β2AR–Gsempty state. The simulations shed light on how the structural elements at the receptor–G-protein interface may interact to transmit the signal over 30 Å to the nucleotide-binding site. Our analysis extends the current limited view of nucleotide-free snapshots to include additional states and structural features responsible for signaling and G-protein coupling specificity. Using molecular dynamics simulations and functional assays, authors track the structural changes in heterotrimeric G proteins in response to receptor coupling that lead to the ejection of GDP, the rate-limiting step during G-protein activation.
G 蛋白与激动剂结合的 G 蛋白偶联受体的机理探究
G 蛋白偶联受体(GPCR)通过促进鸟嘌呤核苷酸交换来激活异三聚体 G 蛋白。在这里,我们研究了 G 蛋白与 GPCR 的耦合,并描述了最终导致 GDP 从 Gα 亚基的结合袋中排出的事件,这是 G 蛋白激活过程中的限速步骤。通过分子动力学模拟,我们研究了与 GDP 结合的 Gs 蛋白(Gs-GDP,以下简称 GsGDP)在与 β2-肾上腺素能受体(β2AR)耦合时结构重排的时间进展细节。GsGDP 与 β2AR 结合后会产生长程异构效应,大大降低 GDP 释放所需的能量:α1-αF 螺旋的打开、αG 螺旋的移位和 α-helical 结构域的打开。信号通过一个扩展的受体界面传播到 Gs,该界面包括位于扭结跨膜螺旋 6 胞内端的一个富含赖氨酸的基团,这一点已通过定点突变和功能测试得到证实。从这个 β2AR-GsGDP 中间体开始,Gs 沿着受体轴进行平面内旋转,以接近 β2AR-Gsempty 状态。模拟揭示了受体-蛋白质界面上的结构元素如何相互作用,将信号传输 30 Å 以上至核苷酸结合位点。我们的分析扩展了目前无核苷酸快照的有限视角,纳入了更多的状态和结构特征,这些状态和特征负责信号传导和 G 蛋白偶联特异性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


