Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
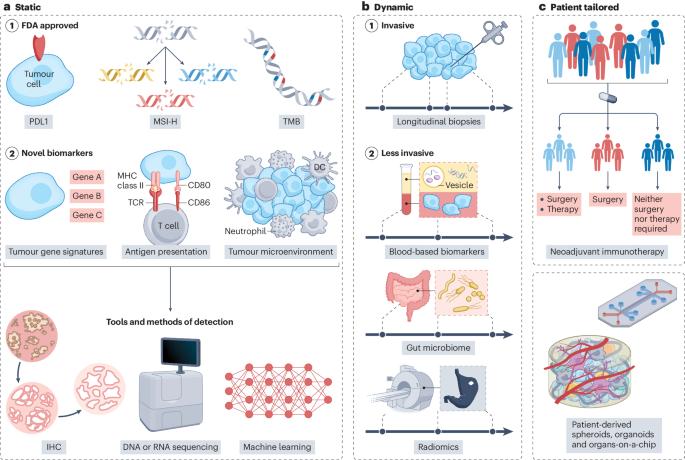
Although more than a decade has passed since the approval of immune checkpoint inhibitors (ICIs) for the treatment of melanoma and non-small-cell lung, breast and gastrointestinal cancers, many patients still show limited response. US Food and Drug Administration (FDA)-approved biomarkers include programmed cell death 1 ligand 1 (PDL1) expression, microsatellite status (that is, microsatellite instability-high (MSI-H)) and tumour mutational burden (TMB), but these have limited utility and/or lack standardized testing approaches for pan-cancer applications. Tissue-based analytes (such as tumour gene signatures, tumour antigen presentation or tumour microenvironment profiles) show a correlation with immune response, but equally, these demonstrate limited efficacy, as they represent a single time point and a single spatial assessment. Patient heterogeneity as well as inter- and intra-tumoural differences across different tissue sites and time points represent substantial challenges for static biomarkers. However, dynamic biomarkers such as longitudinal biopsies or novel, less-invasive markers such as blood-based biomarkers, radiomics and the gut microbiome show increasing potential for the dynamic identification of ICI response, and patient-tailored predictors identified through neoadjuvant trials or novel ex vivo tumour models can help to personalize treatment. In this Perspective, we critically assess the multiple new static, dynamic and patient-specific biomarkers, highlight the newest consortia and trial efforts, and provide recommendations for future clinical trials to make meaningful steps forwards in the field. In this Perspective, Holder et al. discuss the limitations of current predictive biomarkers of response to immune checkpoint inhibitors and the need to further explore static, dynamic and patient-specific biomarkers using novel tools, such as machine learning and consortia-level initiatives.
确定实体瘤免疫检查点抑制剂的临床有用生物标记物
自免疫检查点抑制剂(ICIs)被批准用于治疗黑色素瘤、非小细胞肺癌、乳腺癌和胃肠道癌症以来,十多年过去了,许多患者的反应仍然有限。美国食品和药物管理局(FDA)批准的生物标记物包括程序性细胞死亡配体1(PDL1)表达、微卫星状态(即微卫星不稳定性高(MSI-H))和肿瘤突变负荷(TMB),但这些标记物的效用有限,而且/或者缺乏泛癌症应用的标准化检测方法。基于组织的分析指标(如肿瘤基因特征、肿瘤抗原呈递或肿瘤微环境特征)显示与免疫反应相关,但同样,这些指标的功效有限,因为它们代表的是单一的时间点和单一的空间评估。患者的异质性以及不同组织部位和时间点的肿瘤间和肿瘤内差异是静态生物标记物面临的巨大挑战。然而,纵向活检等动态生物标志物或血液生物标志物、放射组学和肠道微生物组等侵入性较低的新型标志物在动态识别 ICI 反应方面显示出越来越大的潜力,通过新辅助试验或新型体外肿瘤模型确定的适合患者的预测指标有助于实现个性化治疗。在本视角中,我们将对多种新的静态、动态和患者特异性生物标记物进行严格评估,重点介绍最新的联盟和试验工作,并为未来的临床试验提供建议,以便在该领域迈出有意义的一步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


