Synthesis of deoxybenzoins from β-alkoxy styrenes and arylboronic acids via palladium-catalyzed regioselective Heck-arylation reactions†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Palladium-catalyzed synthesis of deoxybenzoin derivatives from styryl ethers and arylboronic acids is reported. The reaction proceeds under mild conditions in the presence of TEMPO and provides the desired products in good to excellent yields. Simple operation, broad substrate scope, and functional group tolerance are the salient features of the developed methodology.
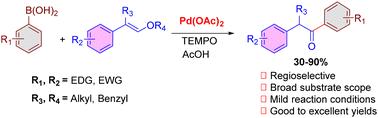
通过钯催化的区域选择性赫克芳基化反应,从 β-烷氧基苯乙烯和芳基硼酸合成脱氧苯并芘。
本研究报道了钯催化合成苯乙烯基醚和芳基硼酸的脱氧苯甲酸衍生物。该反应在 TEMPO 存在下的温和条件下进行,并以良好至极佳的收率提供所需的产物。操作简单、底物范围广和官能团耐受性强是所开发方法的显著特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


