Unravelling the effects of active site density and energetics on the water oxidation activity of iridium oxides
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
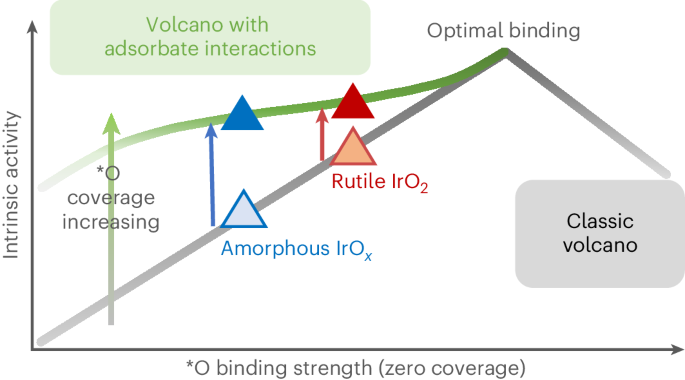
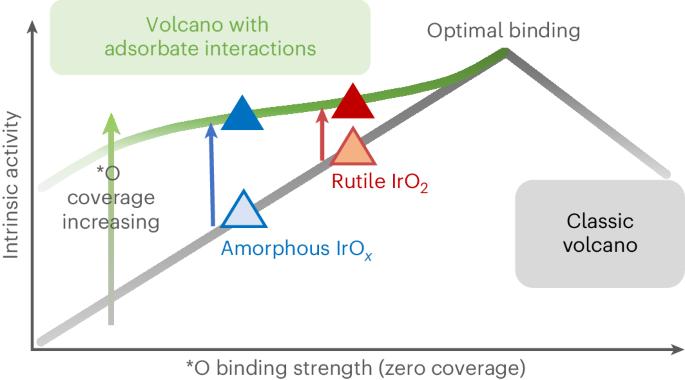
Understanding what controls the reaction rate on iridium-based catalysts is central to designing better electrocatalysts for the water oxidation reaction in proton exchange membrane electrolysers. Here we quantify the densities of redox-active centres and probe their binding strengths on amorphous IrOx and rutile IrO2 using operando time-resolved optical spectroscopy. We establish a quantitative experimental correlation between the intrinsic reaction rate and the active-state energetics. We find that adsorbed oxygen species, *O, formed at water oxidation potentials, exhibit repulsive adsorbate–adsorbate interactions. Increasing their coverage weakens their binding, thereby promoting O–O bond formation, which is the rate-determining step. These analyses suggest that although amorphous IrOx exhibits a higher geometric current density, the intrinsic reaction rates per active state on IrOx and IrO2 are comparable at given potentials. Finally, we present a modified volcano plot that elucidates how the intrinsic water oxidation kinetics can be increased by optimizing both the binding energy and the interaction strength between the catalytically active states. Iridium oxide is the state-of-the-art catalyst for water oxidation in an acidic electrolyte. Now amorphous and crystalline iridium oxides are studied using operando time-resolved optical spectroscopy, together with other techniques, to reveal the nature and density of active centres and the role of adsorbate–adsorbate interactions.
揭示活性位点密度和能量对铱氧化物水氧化活性的影响
要为质子交换膜电解槽中的水氧化反应设计出更好的电催化剂,了解铱基催化剂反应速率的控制因素至关重要。在这里,我们使用操作时间分辨光学光谱法量化了氧化还原活性中心的密度,并探究了它们在无定形氧化铱和金红石氧化铱上的结合强度。我们在固有反应速率和活性状态能量之间建立了定量的实验相关性。我们发现,在水氧化电位下形成的吸附氧物种*O表现出吸附剂与吸附剂之间的排斥性相互作用。增加它们的覆盖率会减弱它们的结合力,从而促进 O-O 键的形成,这是决定速率的一步。这些分析表明,虽然无定形氧化铱表现出更高的几何电流密度,但在给定电位下,氧化铱和二氧化铱上每个活性状态的固有反应速率相当。最后,我们提出了一种改进的火山图,它阐明了如何通过优化催化活性态之间的结合能和相互作用强度来提高固有的水氧化动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


