A de novo designed coiled coil-based switch regulates the microtubule motor kinesin-1
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Many enzymes are allosterically regulated via conformational change; however, our ability to manipulate these structural changes and control function is limited. Here we install a conformational switch for allosteric activation into the kinesin-1 microtubule motor in vitro and in cells. Kinesin-1 is a heterotetramer that accesses open active and closed autoinhibited states. The equilibrium between these states centers on a flexible elbow within a complex coiled-coil architecture. We target the elbow to engineer a closed state that can be opened with a de novo designed peptide. The alternative states are modeled computationally and confirmed by biophysical measurements and electron microscopy. In cells, peptide-driven activation increases kinesin transport, demonstrating a primary role for conformational switching in regulating motor activity. The designs are enabled by our understanding of ubiquitous coiled-coil structures, opening possibilities for controlling other protein activities. The kinesin-1 motor protein accesses open active and closed autoinhibited states. These states are regulated by a flexible elbow within a complex coiled-coil architecture. Now, a conformational switch has been developed by engineering the elbow to create a closed state that can be controllably opened with a de novo designed peptide to increase kinesin transport inside cells.
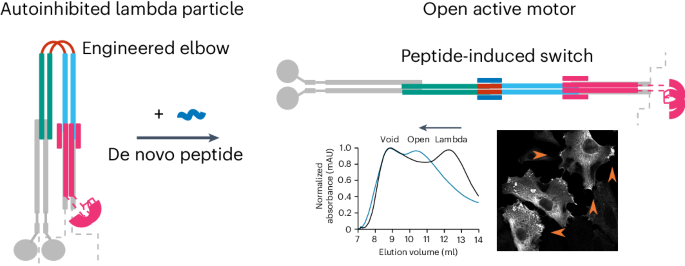
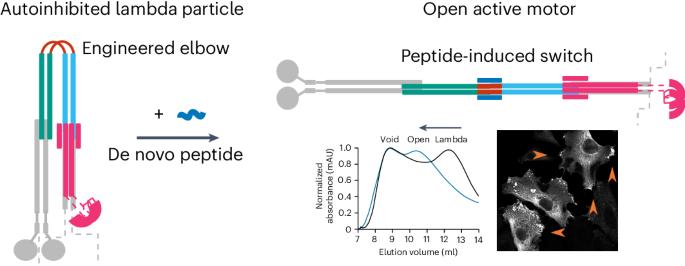
全新设计的基于线圈的开关调控微管马达驱动蛋白-1
许多酶都是通过构象变化进行异构调节的;然而,我们操纵这些结构变化和控制功能的能力却很有限。在这里,我们在体外和细胞中为驱动蛋白-1 微管马达安装了一个构象开关,以实现异位激活。驱动蛋白-1 是一种异源四聚体,可以进入开放的活性状态和封闭的自抑制状态。这些状态之间的平衡集中于一个复杂盘卷结构中的灵活肘部。我们以肘部为目标,设计了一种可通过重新设计的多肽打开的封闭状态。我们通过计算建立了替代状态模型,并通过生物物理测量和电子显微镜进行了确认。在细胞中,多肽驱动的激活增加了驱动蛋白的运输,证明了构象转换在调节马达活动中的主要作用。我们对无处不在的盘绕线圈结构的了解使这些设计成为可能,为控制其他蛋白质的活动提供了可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


