Estrogen receptor β activation alleviates inflammatory bowel disease by suppressing NLRP3-dependent IL-1β production in macrophages via downregulation of intracellular calcium level
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Although several estrogen receptor β (ERβ) agonists have been reported to alleviate IBD, the pivotal mechanism remains obscure.
Objectives
To examine the effects and mechanisms of ERβ activation on cytokine/chemokine networks in colitis mice.
Methods
Dextran sulfate sodium salt (DSS) and trinitro-benzene-sulfonic acid (TNBS) were used to induce mouse colitis model. Multiple molecular biological methods were employed to evaluate the severity of mouse colitis and the level of cytokine and/or chemokine.
Results
Bioinformatics analysis, ELISA and immunofluorescence results showed that the targeted cytokines and/or chemokines associated with ERβ expression and activation is IL-1β, and the anti-colitis effect of ERβ activation was significantly attenuated by the overexpression of AAV9-IL-1β. Immunofluorescence analysis indicated that ERβ activation led to most evident downregulation of IL-1β expression in colonic macrophages as compared to monocytes and neutrophils. Given the pivotal roles of NLRP3, NLRC4, and AIM2 inflammasome activation in the production of IL-1β, we examined the influence of ERβ activation on inflammasome activity. ELISA and WB results showed that ERβ activation selectively blocked the NLRP3 inflammasome assembly-mediated IL-1β secretion. The calcium-sensing receptor (CaSR) and calcium signaling play crucial roles in the assembly of the NLRP3 inflammasome. WB and immunofluorescence results showed that ERβ activation reduced intracellular CaSR expression and calcium signaling in colonic macrophages. Combination with CaSR overexpression plasmid reversed the suppressive effect of ERβ activation on NLRP3 inflammasome assembly, and counteracting the downregulation of IL-1β secretion.
Conclusion
Our research uncovers that the anti-colitis effect of ERβ activation is accomplished through the reduction of IL-1β levels in colonic tissue, achieved by specifically decreasing CaSR expression in macrophages to lower intracellular calcium levels and inhibit NLRP3 inflammasome assembly-mediated IL-1β production.
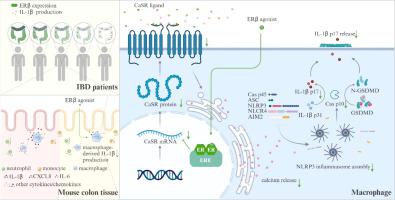

雌激素受体β激活可通过下调细胞内钙水平抑制巨噬细胞中NLRP3依赖性IL-1β的产生,从而缓解炎症性肠病。
简介:尽管有报道称几种雌激素受体 β(ERβ)激动剂可缓解 IBD,但其关键机制仍不明确:尽管有报道称几种雌激素受体β(ERβ)激动剂能缓解IBD,但其关键机制仍不清楚:目的:研究ERβ激活对结肠炎小鼠细胞因子/趋化因子网络的影响和机制:方法:使用葡聚糖硫酸钠盐(DSS)和三硝基苯磺酸(TNBS)诱导小鼠结肠炎模型。采用多种分子生物学方法评估小鼠结肠炎的严重程度以及细胞因子和/或趋化因子的水平:生物信息学分析、ELISA和免疫荧光结果表明,与ERβ表达和活化相关的靶向细胞因子和/或趋化因子是IL-1β,而过表达AAV9-IL-1β会显著减弱ERβ活化的抗结肠炎作用。免疫荧光分析表明,与单核细胞和中性粒细胞相比,ERβ活化导致结肠巨噬细胞中IL-1β表达最明显的下调。鉴于 NLRP3、NLRC4 和 AIM2 炎症小体的激活在 IL-1β 的产生中起着关键作用,我们研究了 ERβ 激活对炎症小体活性的影响。ELISA和WB结果显示,ERβ活化选择性地阻断了NLRP3炎性体组装介导的IL-1β分泌。钙传感受体(CaSR)和钙信号在NLRP3炎性体的组装过程中起着至关重要的作用。WB和免疫荧光结果显示,ERβ激活会降低结肠巨噬细胞内CaSR的表达和钙信号转导。与CaSR过表达质粒结合可逆转ERβ激活对NLRP3炎性体组装的抑制作用,并抵消IL-1β分泌的下调作用:我们的研究发现,ERβ活化的抗结肠炎作用是通过降低结肠组织中的IL-1β水平实现的,而降低IL-1β水平是通过特异性降低巨噬细胞中CaSR的表达,从而降低细胞内钙水平,抑制NLRP3炎性体组装介导的IL-1β分泌。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


