Nucleotide metabolism in cancer cells fuels a UDP-driven macrophage cross-talk, promoting immunosuppression and immunotherapy resistance
IF 28.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
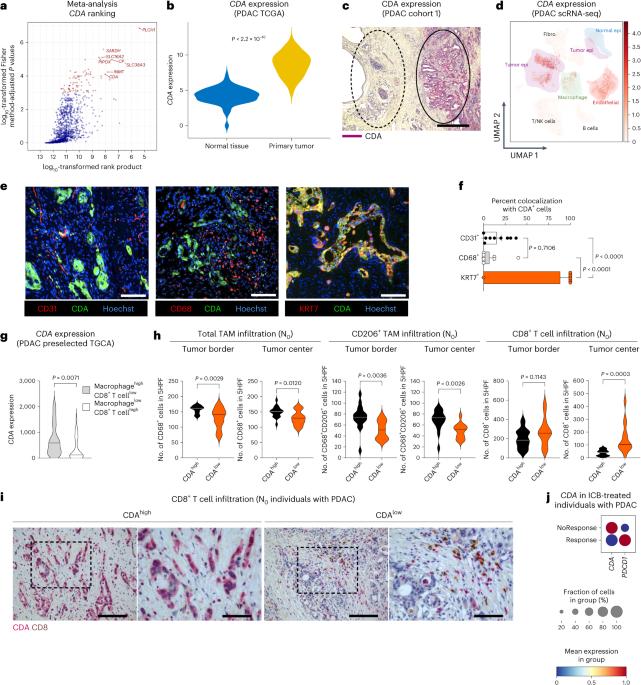
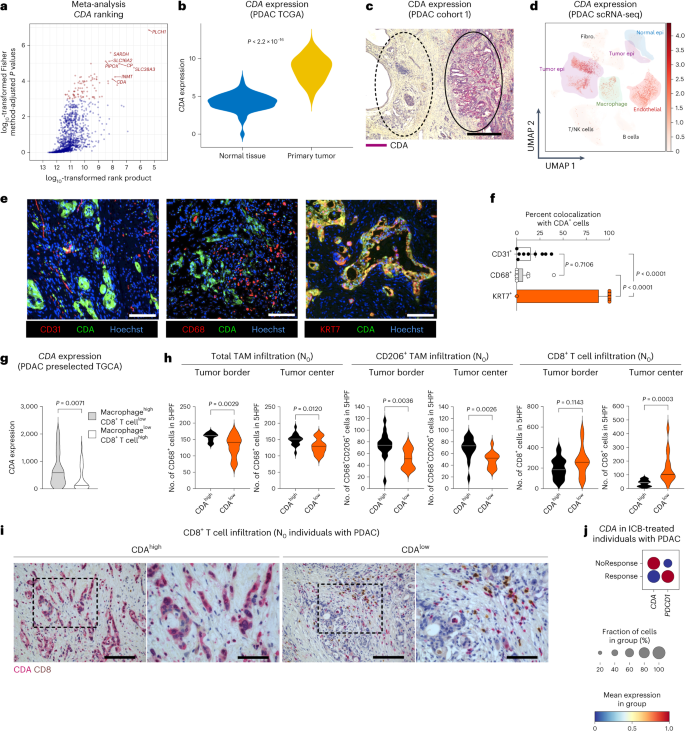
Many individuals with cancer are resistant to immunotherapies. Here, we identify the gene encoding the pyrimidine salvage pathway enzyme cytidine deaminase (CDA) among the top upregulated metabolic genes in several immunotherapy-resistant tumors. We show that CDA in cancer cells contributes to the uridine diphosphate (UDP) pool. Extracellular UDP hijacks immunosuppressive tumor-associated macrophages (TAMs) through its receptor P2Y6. Pharmacologic or genetic inhibition of CDA in cancer cells (or P2Y6 in TAMs) disrupts TAM-mediated immunosuppression, promoting cytotoxic T cell entry and susceptibility to anti-programmed cell death protein 1 (anti-PD-1) treatment in resistant pancreatic ductal adenocarcinoma (PDAC) and melanoma models. Conversely, CDA overexpression in CDA-depleted PDACs or anti-PD-1-responsive colorectal tumors or systemic UDP administration (re)establishes resistance. In individuals with PDAC, high CDA levels in cancer cells correlate with increased TAMs, lower cytotoxic T cells and possibly anti-PD-1 resistance. In a pan-cancer single-cell atlas, CDAhigh cancer cells match with T cell cytotoxicity dysfunction and P2RY6high TAMs. Overall, we suggest CDA and P2Y6 as potential targets for cancer immunotherapy. Scolaro et al. identify the enzyme cytidine deaminase (CDA) as upregulated in immunotherapy-resistant tumors and find it contributes to the UDP pool, which in turn modulates tumor-associated macrophages to instruct an immune-evasive TME.
癌细胞中的核苷酸代谢助长了 UDP 驱动的巨噬细胞交叉对话,促进了免疫抑制和免疫疗法的抗药性。
许多癌症患者对免疫疗法产生抗药性。在这里,我们发现编码嘧啶挽救途径酶胞苷脱氨酶(CDA)的基因是几种免疫疗法耐药肿瘤中最高调的代谢基因之一。我们发现,癌细胞中的 CDA 对二磷酸尿苷(UDP)池有贡献。细胞外 UDP 通过其受体 P2Y6 劫持具有免疫抑制作用的肿瘤相关巨噬细胞(TAMs)。在抗药性胰腺导管腺癌(PDAC)和黑色素瘤模型中,对癌细胞中的 CDA(或 TAMs 中的 P2Y6)进行药物或基因抑制会破坏 TAM 介导的免疫抑制,促进细胞毒性 T 细胞进入,并使其易受抗程序性细胞死亡蛋白 1(anti-PD-1)治疗的影响。相反,在 CDA 贫乏的 PDAC 或抗 PD-1 反应性结直肠肿瘤中 CDA 过表达或全身 UDP 给药会(重新)建立抗药性。在 PDAC 患者中,癌细胞中 CDA 水平高与 TAMs 增加、细胞毒性 T 细胞减少以及可能的抗 PD-1 抗性相关。在泛癌症单细胞图谱中,CDA水平高的癌细胞与T细胞细胞毒性功能障碍和P2RY6水平高的TAMs相匹配。总之,我们建议将 CDA 和 P2Y6 作为癌症免疫疗法的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


