Significantly enhanced performance for phenol compounds removal by MOF-5 nano-composite via its surface modification
IF 10.4
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
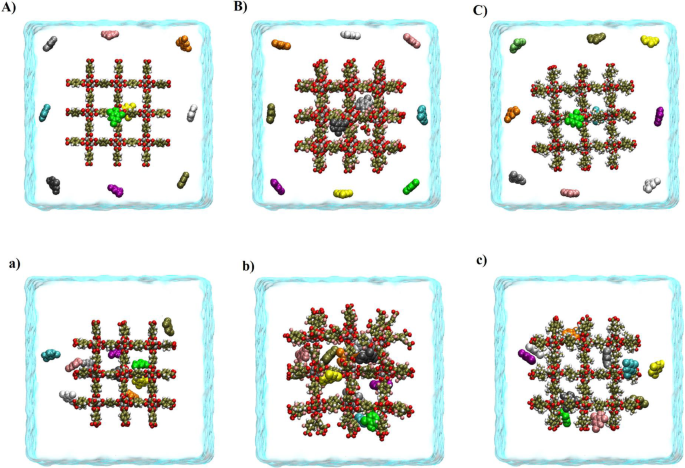
The present study is focused on the use of cubic metal-organic frameworks-5 (MOF-5) and its functionalized form in the removal of phenolic pollutants by molecular dynamics (MD) and Well-tempered metadynamics (WTMD) simulation methods. It was found that the adsorption mechanism of MOF-5s/phenolic compounds is mostly due to the van der Waals and π–π interactions. However, electrostatic and hydrogen bond (HB) interactions also play a significant role in removing phenolic pollutants by MOF-5 and its functionalized form. The results show that the fluorine functional group (F-MOF-5) increases the adsorption capacity of phenol compounds on the adsorbent surface. By functionalizing the MOF-5 with a methyl functional group (CH3-MOF-5), the adsorption strength decreases. The WTMD calculation confirmed that at the most stable state, the free energy (FE) value of system II (the most stable system in functionalized systems with –F functional group) is about −289.528 kJ mol−1. This value is ~5.781 and 35.514 kJ mol−1 more negative than the FE of the I and III systems (the most stable systems in the pristine and CH3-MOF-5/pollutant systems, respectively). Altogether, the results indicate that F-MOF-5 can be considered a more suitable adsorbent than MOF-5 and CH3-MOF-5 for phenolic pollutants removal from the environment for more assessment.
通过表面改性,MOF-5 纳米复合材料去除苯酚化合物的性能显著提高
本研究通过分子动力学(MD)和井态元动力学(WTMD)模拟方法,重点研究了立方金属有机框架-5(MOF-5)及其功能化形式在去除酚类污染物中的应用。研究发现,MOF-5/酚类化合物的吸附机理主要是由于范德华和π-π相互作用。然而,静电和氢键(HB)相互作用也在 MOF-5 及其官能化形式去除酚类污染物的过程中发挥了重要作用。结果表明,氟官能团(F-MOF-5)增加了苯酚化合物在吸附剂表面的吸附能力。用甲基官能团(CH3-MOF-5)对 MOF-5 进行官能化后,吸附强度会降低。WTMD 计算证实,在最稳定的状态下,体系 II(带 -F 官能团的官能化体系中最稳定的体系)的自由能(FE)值约为 -289.528 kJ mol-1。该值比 I 和 III 系统(分别是原始系统和 CH3-MOF-5/ 污染物系统中最稳定的系统)的自由能负值分别高出约 5.781 和 35.514 kJ mol-1。总之,研究结果表明,与 MOF-5 和 CH3-MOF-5 相比,F-MOF-5 更适合作为一种吸附剂,用于从环境中去除酚类污染物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

npj Clean Water
Environmental Science-Water Science and Technology
CiteScore
15.30
自引率
2.60%
发文量
61
审稿时长
5 weeks
期刊介绍:
npj Clean Water publishes high-quality papers that report cutting-edge science, technology, applications, policies, and societal issues contributing to a more sustainable supply of clean water. The journal's publications may also support and accelerate the achievement of Sustainable Development Goal 6, which focuses on clean water and sanitation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


