Rh(iii)-catalyzed atroposelective C–H alkynylation of 1-aryl isoquinolines with hypervalent iodine–alkyne reagents†
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An efficient Rh(iii)-catalyzed enantioselective C–H alkynylation of isoquinolines is disclosed. The C–H alkynylation of 1-aryl isoquinolines with hypervalent iodine–alkyne reagents proceeded in DMA at room temperature in the presence of 2.5 mol% chiral SCpRh(iii) complex along with 20 mol% AgSbF6, providing axially chiral alkynylated 1-aryl isoquinolines in excellent yields (up to 93%) and enantioselectivity (up to 95% ee). The diverse transformations of the product further enhance the potential utility of this reaction.
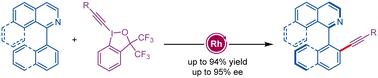
Rh(III)-Catalyzed Atroposelective C-H Alkynylation of 1-Aryl Isoquinolines with Hypervalent Iodine-Alkyne Reagents(Rh(III)催化的高价碘-炔试剂对 1-芳基异喹啉的无选择性 C-H 烷基化反应
本研究揭示了一种高效的 Rh(III) 催化的异喹啉类对映体选择性 C-H 烷炔化反应。在 2.5 mol% 手性 SCpRh(III) 复合物和 20 mol% AgSbF4 的存在下,1-芳基异喹啉与高价碘-炔试剂在室温下于 DMA 中进行 C-H 烷炔化反应,以优异的收率(高达 93%)和对映选择性(高达 95% ee)提供了轴向手性烷炔化的 1-芳基异喹啉。产物的多种转化进一步增强了该反应的潜在用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


