Gas-balancing adsorption strategy towards noble-metal-based nanowire electrocatalysts
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The preparation of noble metal nanowire electrocatalysts is greatly limited by the thermodynamically symmetric growth of face-centred-cubic structures. Here we report a gas-balancing adsorption strategy to prepare ultrathin palladium-, platinum- and gold-based nanowires (diameter < 2 nm) by controlling the competitive adsorption of in situ-generated H2 and CO. We prepare a library of 43 nanowires consisting of the three above-mentioned noble metals as hosts and 14 metals as guests. The ternary Pd85Pt8Ni7H41 nanowires with interstitial hydrogen exhibit impressive mass and specific activities of $$11.1 \, {\rm{A}}\,{\rm{mg}}_{{\rm{PGM}}}^{-1}$$ and 13.9 mA cm−2, respectively, for the oxygen reduction reaction at 0.9 VRHE in alkali. Operando X-ray absorption spectroscopy demonstrates breathing-like Pd–Pd bond length and strain changes at the applied potential, with Pd85Pt8Ni7H41 nanowires exhibiting larger compressive strain at relevant potentials, as well as low oxygen coverage. Theoretical calculations suggest that the interstitial hydrogen induces an s–d orbital interaction between palladium and hydrogen, which enhances the activity of the oxygen reduction reaction. The Pd85Pt8Ni7H41 nanowires can generate a high power density of 0.87 W cm−2 in H2/air (CO2-free) at 70 °C in an anion-exchange membrane fuel cell. Nanostructured design of mono- and multimetallic particles can be leveraged to achieve highly active catalysts. Now, a gas-balancing adsorption strategy is presented to prepare alloy nanowires with diameter of around 1 nm, whereby resulting catalysts achieve excellent performance for both anion- and proton-exhange membrane fuel cells.
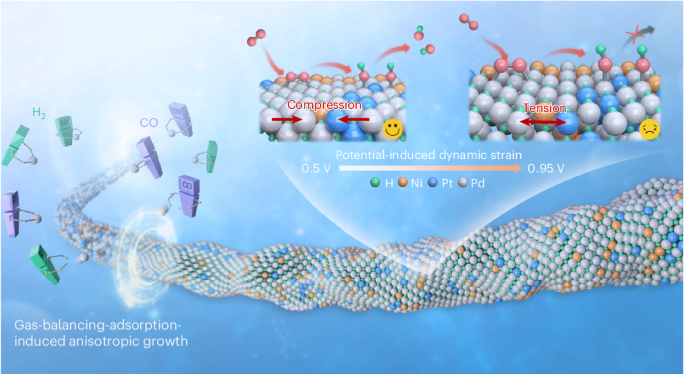
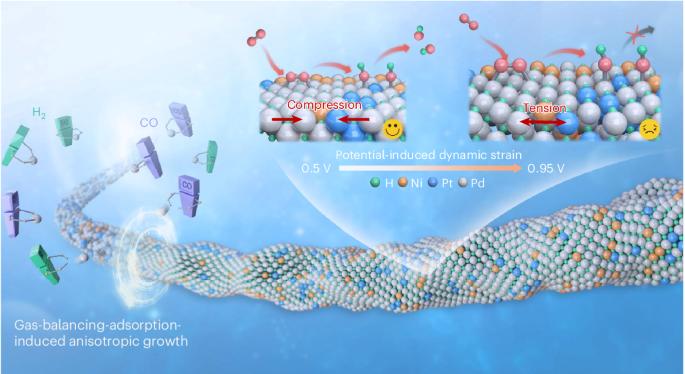
贵金属纳米线电催化剂的气体平衡吸附策略
贵金属纳米线电催化剂的制备受到面心立方体结构热力学对称生长的极大限制。在此,我们报告了一种气体平衡吸附策略,通过控制原位生成的 H2 和 CO 的竞争性吸附,制备超薄钯基、铂基和金基纳米线(直径为 2 nm)。我们制备了由上述三种贵金属作为主金属和 14 种金属作为客金属组成的 43 种纳米线。含有间隙氢的三元 Pd85Pt8Ni7H41 纳米线在 0.9 VRHE 碱条件下的氧还原反应中表现出令人印象深刻的质量活性和比活性,分别为 11.1 \, {\rm{A}}\, {\rm{mg}}_{{\rm{PGM}}^{-1}\ 和 13.9 mA cm-2。操作性 X 射线吸收光谱显示,在施加的电位下,Pd-Pd 键的长度和应变发生了类似呼吸的变化,Pd85Pt8Ni7H41 纳米线在相关电位下表现出较大的压缩应变以及较低的氧覆盖率。理论计算表明,间隙氢诱导了钯与氢之间的 s-d 轨道相互作用,从而提高了氧还原反应的活性。在阴离子交换膜燃料电池中,Pd85Pt8Ni7H41 纳米线能在 70 °C 下的 H2/空气(无二氧化碳)中产生 0.87 W cm-2 的高功率密度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


