Global profiling of functional histidines in live cells using small-molecule photosensitizer and chemical probe relay labelling
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Recent advances in chemical proteomics have focused on developing chemical probes that react with nucleophilic amino acid residues. Although histidine is an attractive candidate due to its importance in enzymatic catalysis, metal binding and protein–protein interaction, its moderate nucleophilicity poses challenges. Its modification is frequently influenced by cysteine and lysine, which results in poor selectivity and narrow proteome coverage. Here we report a singlet oxygen and chemical probe relay labelling method that achieves high selectivity towards histidine. Libraries of small-molecule photosensitizers and chemical probes were screened to optimize histidine labelling, enabling histidine profiling in live cells with around 7,200 unique sites. Using NMR spectroscopy and X-ray crystallography, we characterized the reaction mechanism and the structures of the resulting products. We then applied this method to discover unannotated histidine sites key to enzymatic activity and metal binding in select metalloproteins. This method also revealed the accessibility change of histidine mediated by protein–protein interaction that influences select protein subcellular localization, underscoring its capability in discovering functional histidines. Chemical probes that selectively react with histidine could afford functional insight for those located in vital protein regions, but the moderate nucleophilicity of histidine and interference from other residues pose challenges. A singlet oxygen and chemical probe relay labelling approach demonstrates high selectivity, enabling comprehensive histidine profiling and providing crucial functional insights.
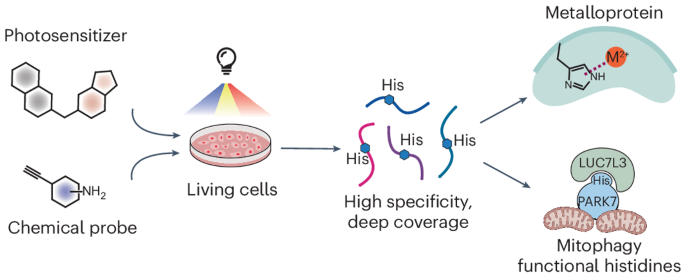
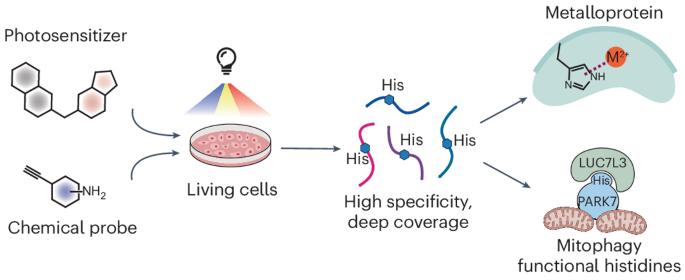
利用小分子光敏剂和化学探针中继标记对活细胞中的功能组氨酸进行全局分析
化学蛋白质组学的最新进展主要集中在开发能与亲核氨基酸残基发生反应的化学探针上。虽然组氨酸在酶催化、金属结合和蛋白质-蛋白质相互作用中具有重要作用,因此是一种有吸引力的候选物质,但其适度的亲核性也带来了挑战。组氨酸的修饰经常受到半胱氨酸和赖氨酸的影响,从而导致选择性差和蛋白质组覆盖范围窄。在此,我们报告了一种单线态氧和化学探针接力标记方法,该方法对组氨酸具有高选择性。我们筛选了小分子光敏剂和化学探针库,以优化组氨酸标记,从而实现了活细胞中约 7,200 个独特位点的组氨酸分析。利用核磁共振光谱和 X 射线晶体学,我们确定了反应机制和生成物的结构。然后,我们应用这种方法发现了未注明的组氨酸位点,这些位点是酶活性和金属结合的关键。该方法还揭示了组氨酸在蛋白质与蛋白质相互作用过程中发生的可及性变化,这种变化影响了特定蛋白质的亚细胞定位,从而凸显了该方法在发现功能组氨酸方面的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


