XIST dampens X chromosome activity in a SPEN-dependent manner during early human development
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
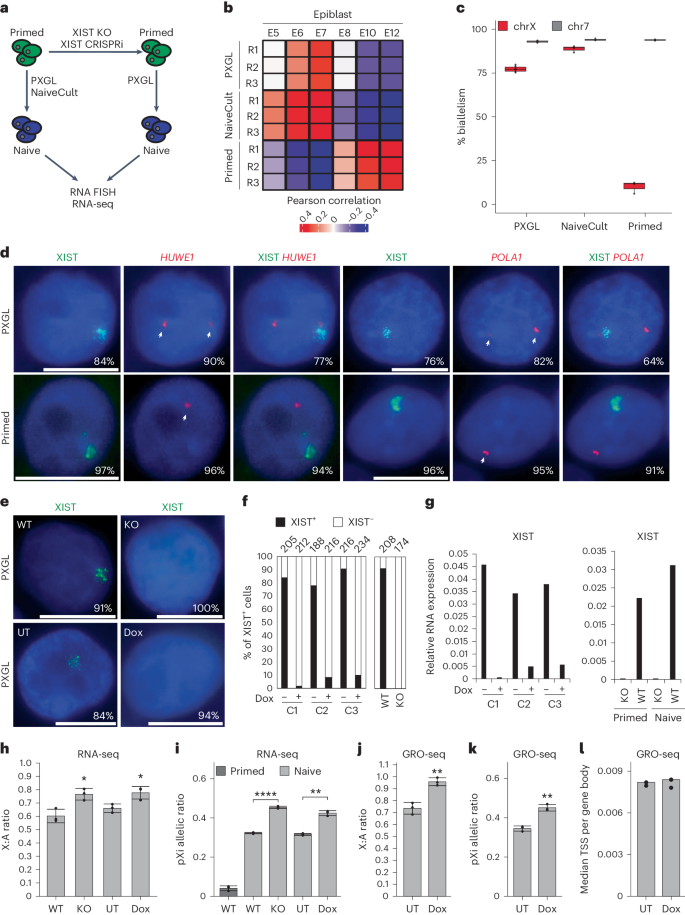
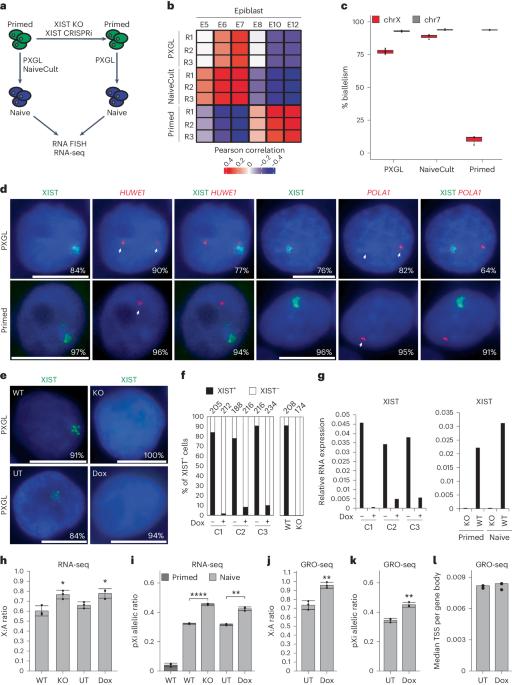
XIST (X-inactive specific transcript) long noncoding RNA (lncRNA) is responsible for X chromosome inactivation (XCI) in placental mammals, yet it accumulates on both X chromosomes in human female preimplantation embryos without triggering X chromosome silencing. The XACT (X-active coating transcript) lncRNA coaccumulates with XIST on active X chromosomes and may antagonize XIST function. Here, we used human embryonic stem cells in a naive state of pluripotency to assess the function of XIST and XACT in shaping the X chromosome chromatin and transcriptional landscapes during preimplantation development. We show that XIST triggers the deposition of polycomb-mediated repressive histone modifications and dampens the transcription of most X-linked genes in a SPEN-dependent manner, while XACT deficiency does not significantly affect XIST activity or X-linked gene expression. Our study demonstrates that XIST is functional before XCI, confirms the existence of a transient process of X chromosome dosage compensation and reveals that XCI and dampening rely on the same set of factors. Using naive human embryonic stem cells as a model for early embryogenesis, the authors report that the XIST (X-inactive specific transcript) long noncoding RNA recruits repressive histone marks and attenuates X chromosome expression before the establishment of X chromosome inactivation.
在人类早期发育过程中,XIST 以依赖 SPEN 的方式抑制 X 染色体的活性
XIST(X-inactive specific transcript)长非编码 RNA(lncRNA)在胎盘哺乳动物中负责 X 染色体失活(XCI),但它会在人类女性植入前胚胎的两条 X 染色体上积累,而不会引发 X 染色体沉默。XACT(X-活性包被转录本)lncRNA与XIST共同聚集在活性X染色体上,可能会拮抗XIST的功能。在这里,我们利用处于幼稚多能状态的人类胚胎干细胞,评估了XIST和XACT在植入前发育过程中塑造X染色体染色质和转录景观的功能。我们的研究表明,XIST 会触发多聚酶介导的抑制性组蛋白修饰沉积,并以一种依赖 SPEN 的方式抑制大多数 X 连锁基因的转录,而 XACT 的缺乏并不会显著影响 XIST 的活性或 X 连锁基因的表达。我们的研究表明,XIST 在 XCI 之前就已发挥作用,证实了 X 染色体剂量补偿过程的存在,并揭示了 XCI 和抑制作用依赖于同一组因子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


