Envisioning how to advance the MASH field
IF 45.9
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
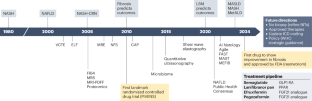
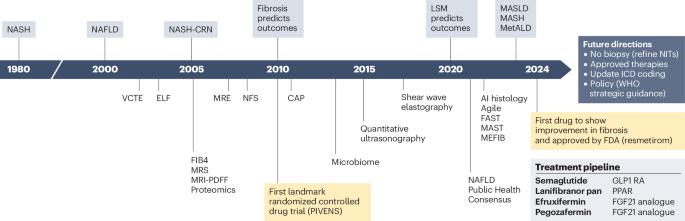
Since 1980, the cumulative effort of scientists and health-care stakeholders has advanced the prerequisites to address metabolic dysfunction-associated steatotic liver disease (MASLD), a prevalent chronic non-communicable liver disease. This effort has led to, among others, the approval of the first drug specific for metabolic dysfunction-associated steatohepatitis (MASH; formerly known as nonalcoholic steatohepatitis). Despite substantial progress, MASLD is still a leading cause of advanced chronic liver disease, including primary liver cancer. This Perspective contextualizes the nomenclature change from nonalcoholic fatty liver disease to MASLD and proposes important considerations to accelerate further progress in the field, optimize patient-centric multidisciplinary care pathways, advance pharmacological, behavioural and diagnostic research, and address health disparities. Key regulatory and other steps necessary to optimize the approval and access to upcoming additional pharmacological therapeutic agents for MASH are also outlined. We conclude by calling for increased education and awareness, enhanced health system preparedness, and concerted action by policy-makers to further the public health and policy agenda to achieve at least parity with other non-communicable diseases and to aid in growing the community of practice to reduce the human and economic burden and end the public health threat of MASLD and MASH by 2030. This Perspective discusses the nomenclature change from nonalcoholic fatty liver disease to metabolic dysfunction-associated steatotic liver disease (MASLD) and proposes steps necessary to improve care and end the public health threat posed by MASLD and metabolic dysfunction-associated steatohepatitis.
设想如何推动 MASH 领域的发展
自 1980 年以来,科学家和医疗保健领域的相关人士不断努力,为解决代谢功能障碍相关性脂肪性肝病(MASLD)这一普遍存在的慢性非传染性肝病提供了先决条件。这项工作的成果之一是批准了第一种治疗代谢功能障碍相关性脂肪性肝炎(MASH,以前称为非酒精性脂肪性肝炎)的特效药。尽管取得了重大进展,但代谢紊乱性脂肪性肝炎仍是导致晚期慢性肝病(包括原发性肝癌)的主要原因。本视角介绍了将非酒精性脂肪肝更名为 MASLD 的背景,并提出了一些重要的考虑因素,以加快该领域的进一步进展,优化以患者为中心的多学科护理途径,推进药物、行为和诊断研究,并解决健康差异问题。此外,我们还概述了为优化即将批准和使用的其他 MASH 药物治疗所需的关键监管和其他步骤。最后,我们呼吁加强教育和宣传,增强卫生系统的准备工作,并呼吁决策者采取一致行动,推进公共卫生和政策议程,至少实现与其他非传染性疾病的平等,并帮助扩大实践社区,以减轻人力和经济负担,到 2030 年消除 MASLD 和 MASH 对公共卫生的威胁。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
52.30
自引率
0.60%
发文量
147
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Gastroenterology & Hepatology aims to serve as the leading resource for Reviews and commentaries within the scientific and medical communities it caters to. The journal strives to maintain authority, accessibility, and clarity in its published articles, which are complemented by easily understandable figures, tables, and other display items. Dedicated to providing exceptional service to authors, referees, and readers, the editorial team works diligently to maximize the usefulness and impact of each publication.
The journal encompasses a wide range of content types, including Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements, all pertinent to gastroenterologists and hepatologists. With its broad scope, Nature Reviews Gastroenterology & Hepatology ensures that its articles reach a diverse audience, aiming for the widest possible dissemination of valuable information.
Nature Reviews Gastroenterology & Hepatology is part of the Nature Reviews portfolio of journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


