Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
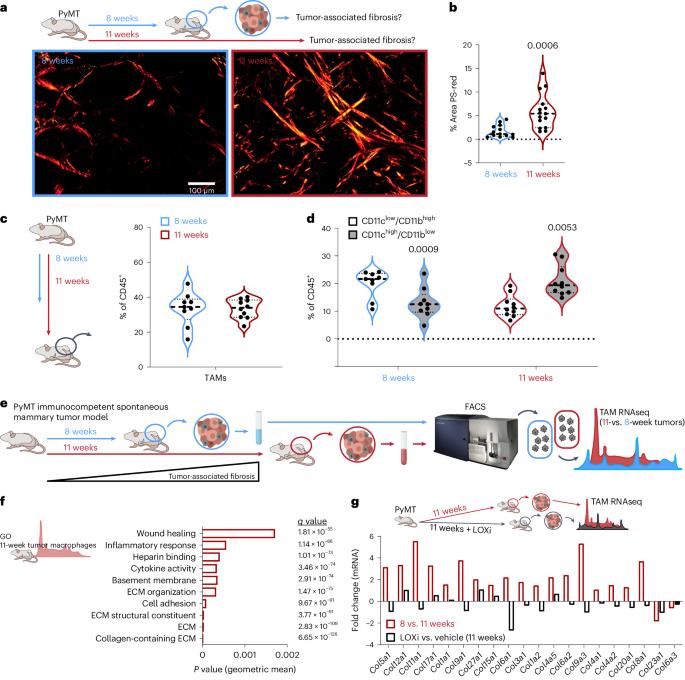
Tumor progression is accompanied by fibrosis, a condition of excessive extracellular matrix accumulation, which is associated with diminished antitumor immune infiltration. Here we demonstrate that tumor-associated macrophages (TAMs) respond to the stiffened fibrotic tumor microenvironment (TME) by initiating a collagen biosynthesis program directed by transforming growth factor-β. A collateral effect of this programming is an untenable metabolic milieu for productive CD8+ T cell antitumor responses, as collagen-synthesizing macrophages consume environmental arginine, synthesize proline and secrete ornithine that compromises CD8+ T cell function in female breast cancer. Thus, a stiff and fibrotic TME may impede antitumor immunity not only by direct physical exclusion of CD8+ T cells but also through secondary effects of a mechano-metabolic programming of TAMs, which creates an inhospitable metabolic milieu for CD8+ T cells to respond to anticancer immunotherapies. Weaver and colleagues show that TGFβ-induced collagen deposition and metabolic reprogramming of the breast cancer microenvironment by tumor-associated macrophages restrict the antitumor activity of CD8+ T cells in female breast cancer.
肿瘤相关巨噬细胞通过胶原沉积和乳腺癌微环境的代谢重编程限制 CD8+ T 细胞的功能。
肿瘤进展伴随着纤维化,这是一种细胞外基质过度积累的情况,与抗肿瘤免疫浸润减少有关。在这里,我们证明了肿瘤相关巨噬细胞(TAMs)通过启动由转化生长因子-β引导的胶原蛋白生物合成程序来应对僵化的纤维化肿瘤微环境(TME)。由于胶原合成巨噬细胞会消耗环境中的精氨酸、合成脯氨酸并分泌鸟氨酸,从而损害了 CD8+ T 细胞在女性乳腺癌中的功能。因此,僵硬和纤维化的 TME 可能不仅通过直接物理排斥 CD8+ T 细胞,而且还通过 TAMs 机械代谢程序的继发效应阻碍抗肿瘤免疫,这为 CD8+ T 细胞对抗癌免疫疗法做出反应创造了一个不适宜的代谢环境。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


