Counterintuitive chemoselectivity in the reduction of carbonyl compounds
IF 51.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The reactivity of carbonyl functional groups largely depends on the substituents on the carbon atom. Reversal of the commonly accepted order of reactivity of different carbonyl compounds requires novel synthetic approaches. Achieving selective reduction will enable the transformation of carbon resources such as plastic waste, carbon dioxide and biomass into valuable chemicals. In this Review, we explore the reduction of less reactive carbonyl groups in the presence of those typically considered more reactive. We discuss reductions, including the controlled reduction of ureas, amides and esters to aldehydes, as well as chemoselective reductions of carbonyl groups, including the reduction of ureas over carbamates, amides and esters; the reduction of amides over esters, ketones and aldehydes; and the reduction of ketones over aldehydes. Reversing the intuitive order of reactivity of functional groups provides new synthetic strategies and enables utilization of chemical feedstocks, such as plastic waste, carbon dioxide and biomass. This Review highlights the chemoselective reduction of carbonyl compounds with a counterintuitive reactivity order.
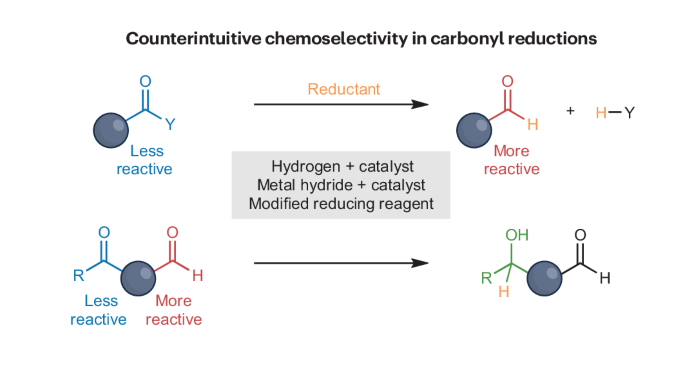
羰基化合物还原过程中的反直觉化学选择性。
羰基官能团的反应性在很大程度上取决于碳原子上的取代基。要扭转不同羰基化合物普遍接受的反应顺序,需要采用新的合成方法。实现选择性还原将使塑料废料、二氧化碳和生物质等碳资源转化为有价值的化学品。在本综述中,我们将探讨在通常被认为反应性较高的羰基存在的情况下还原反应性较低的羰基。我们讨论的还原方法包括将脲基、酰胺和酯受控还原成醛,以及羰基的化学选择性还原,包括脲基还原氨基甲酸酯、酰胺和酯;酰胺还原酯、酮和醛;以及酮还原醛。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


