Reversing the charge of lysine by genetic code expansion
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Posttranslational modifications alter the structure and function of proteins. Now, genetic code expansion enables encoding of ε-N-succinyllysine and ε-N-glutaryllysine residues to decipher the effects of these modifications on enzymatic activity, protein–protein interactions and protein–DNA interactions.
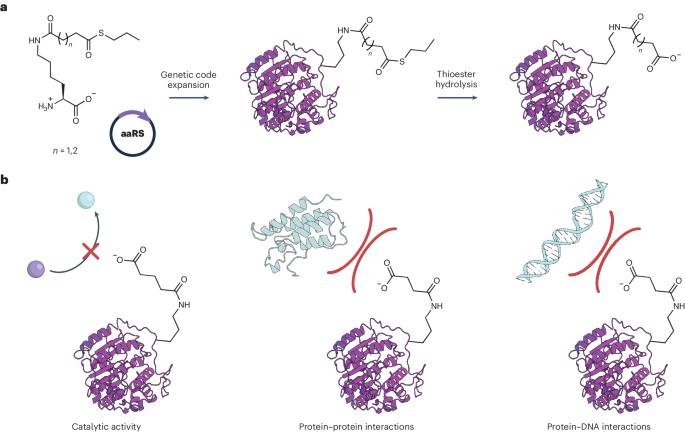
通过扩展遗传密码扭转赖氨酸的电荷
翻译后修饰会改变蛋白质的结构和功能。现在,通过扩展遗传密码,可以对ε-N-琥珀酰赖氨酸和ε-N-谷氨酰赖氨酸残基进行编码,从而破译这些修饰对酶活性、蛋白质-蛋白质相互作用以及蛋白质-DNA相互作用的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


