Fucoidan alleviates high sucrose-induced metabolic disorders and enhances intestinal homeostasis through modulation of Notch signaling
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
The therapeutic potential of fucoidan (FUC), a natural polysaccharide, in metabolic disorders is recognized, yet its underlying mechanisms remain unclear.
Methods
We conducted investigations into the therapeutic mechanisms of FUC sourced from Sargassum fulvellum concerning metabolic disorders induced by a high-sucrose diet (HSD), employing Drosophila melanogaster and mice models. Drosophila larvae were subjected to HSD exposure to monitor growth inhibition, reduced pupation, and developmental delays. Additionally, we examined the impact of FUC on growth- and development-related hormones in Drosophila. Furthermore, we assessed the modulation of larval intestinal homeostasis by FUC, focusing on the regulation of Notch signaling. In mice, we evaluated the effects of FUC on HSD-induced impairments in intestinal epithelial barrier integrity and gut hormone secretion.
Results
FUC supplementation significantly enhanced pupal weight in Drosophila larvae and effectively countered HSD-induced elevation of glucose and triglyceride levels. It notably influenced the expression of growth- and development-related hormones, particularly augmenting insulin-like peptides production while mitigating larval growth retardation. FUC also modulated larval intestinal homeostasis by negatively regulating Notch signaling, thereby protecting against HSD-induced metabolic stress. In mice, FUC ameliorated HSD-induced impairments in ileum epithelial barrier integrity and gut hormone secretion.
Conclusions
Our findings demonstrate the multifaceted therapeutic effects of FUC in mitigating metabolic disorders and maintaining intestinal health. FUC holds promise as a therapeutic agent, with its effects attributed partly to the sulfate group and its ability to regulate Notch signaling, emphasizing its potential for addressing metabolic disorders.
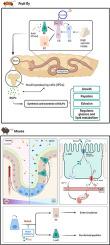

褐藻糖胶通过调节Notch信号传导缓解高蔗糖诱导的代谢紊乱并增强肠道稳态。
导言:褐藻糖胶(FUC)是一种天然多糖,其对代谢紊乱的治疗潜力已得到认可,但其潜在机制仍不清楚:方法:我们利用黑腹果蝇和小鼠模型,研究了来自马尾藻的褐藻糖胶对高蔗糖饮食(HSD)引起的代谢紊乱的治疗机制。果蝇幼虫暴露于高蔗糖饮食(HSD)后,会出现生长抑制、化蛹减少和发育迟缓。此外,我们还研究了FUC对果蝇生长和发育相关激素的影响。此外,我们还评估了 FUC 对幼虫肠道稳态的调节作用,重点关注 Notch 信号的调节。在小鼠中,我们评估了FUC对HSD诱导的肠上皮屏障完整性和肠道激素分泌损伤的影响:结果:补充 FUC 能明显增加果蝇幼虫的蛹重,并有效抑制 HSD 诱导的葡萄糖和甘油三酯水平的升高。它显著影响了生长和发育相关激素的表达,特别是增加了胰岛素样肽的分泌,同时缓解了幼虫生长迟缓。FUC 还通过负向调节 Notch 信号来调节幼虫肠道稳态,从而保护幼虫免受 HSD 诱导的代谢压力的影响。在小鼠体内,FUC 可改善 HSD 诱导的回肠上皮屏障完整性和肠道激素分泌损伤:我们的研究结果表明了 FUC 在缓解代谢紊乱和维护肠道健康方面的多方面治疗效果。FUC有望成为一种治疗药物,其作用部分归因于硫酸基团及其调节Notch信号转导的能力,这强调了它在解决代谢紊乱方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


