METTL3-mediated pre-miR-665/DLX3 m6A methylation facilitates the committed differentiation of stem cells from apical papilla
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
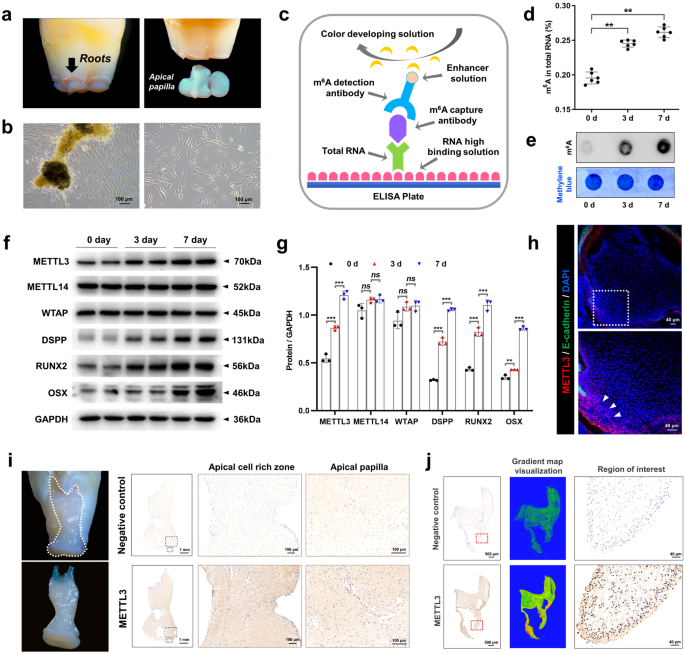
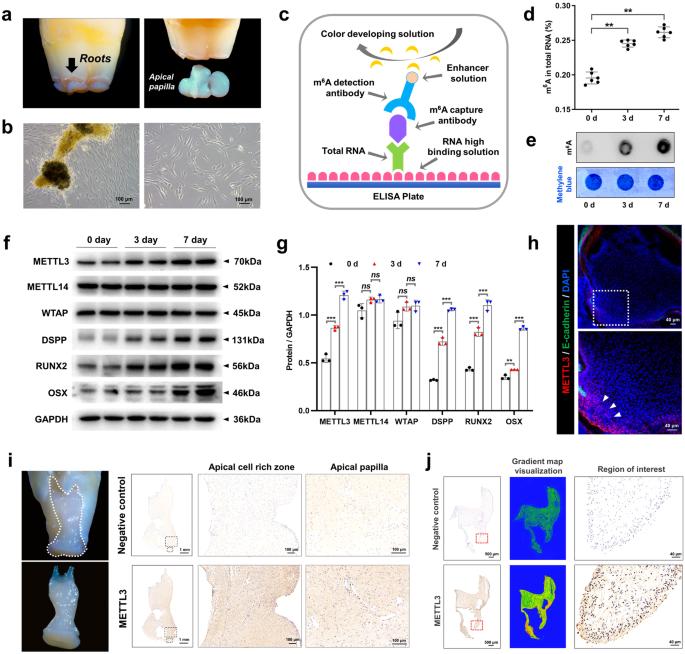
Methyltransferase-like 3 (METTL3) is a crucial element of N6-methyladenosine (m6A) modifications and has been extensively studied for its involvement in diverse biological and pathological processes. In this study, we explored how METTL3 affects the differentiation of stem cells from the apical papilla (SCAPs) into odonto/osteoblastic lineages through gain- and loss-of-function experiments. The m6A modification levels were assessed using m6A dot blot and activity quantification experiments. In addition, we employed Me-RIP microarray experiments to identify specific targets modified by METTL3. Furthermore, we elucidated the molecular mechanism underlying METTL3 function through dual-luciferase reporter gene experiments and rescue experiments. Our findings indicated that METTL3+/− mice exhibited significant root dysplasia and increased bone loss. The m6A level and odonto/osteoblastic differentiation capacity were affected by the overexpression or inhibition of METTL3. This effect was attributed to the acceleration of pre-miR-665 degradation by METTL3-mediated m6A methylation in cooperation with the “reader” protein YTHDF2. Additionally, the targeting of distal-less homeobox 3 (DLX3) by miR-665 and the potential direct regulation of DLX3 expression by METTL3, mediated by the “reader” protein YTHDF1, were demonstrated. Overall, the METTL3/pre-miR-665/DLX3 pathway might provide a new target for SCAP-based tooth root/maxillofacial bone tissue regeneration. Dentition defects, or missing teeth, can cause long-term health issues and are typically treated with implant denture restoration, a method that often has complications. Recent studies suggest using dental stem cells for treatment. A study by Nanjing Medical University investigated the role of a molecule, METTL3, in the development of stem cells from the apical papilla (SCAPs), a type of dental stem cell. They discovered that METTL3 helps SCAPs develop into odonto/osteogenic cells, important for tooth and bone growth. This implies that METTL3 could enhance treatments for missing teeth. The study also found a regulatory network involving METTL3 and other molecules, which could be significant in future research. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
METTL3介导的前miR-665/DLX3 m6A甲基化促进了顶端乳头干细胞的坚定分化。
甲基转移酶样3(METTL3)是N6-甲基腺苷(m6A)修饰的关键因素,因其参与多种生物和病理过程而被广泛研究。在这项研究中,我们通过功能增益和功能缺失实验,探讨了METTL3如何影响顶端乳头干细胞(SCAPs)分化为骨发育系/骨细胞系。m6A点印迹和活性定量实验评估了m6A修饰水平。此外,我们还利用 Me-RIP 微阵列实验来确定 METTL3 修饰的特定靶标。此外,我们还通过双荧光素酶报告基因实验和拯救实验阐明了METTL3功能的分子机制。我们的研究结果表明,METTL3+/-小鼠表现出明显的牙根发育不良和骨质流失。过表达或抑制 METTL3 会影响 m6A 水平和颌骨/骨细胞分化能力。这种影响归因于 METTL3 与 "阅读器 "蛋白 YTHDF2 合作介导的 m6A 甲基化加速了前 miR-665 的降解。此外,研究还证明了 miR-665 对无远端同源染色体 3(DLX3)的靶向作用,以及 METTL3 在 "阅读器 "蛋白 YTHDF1 的介导下对 DLX3 表达的潜在直接调控。总之,METTL3/pre-miR-665/DLX3通路可能为基于SCAP的牙根/颌面骨组织再生提供一个新的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


