Dissecting the MUC5AC/ANXA2 signaling axis: implications for brain metastasis in lung adenocarcinoma
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
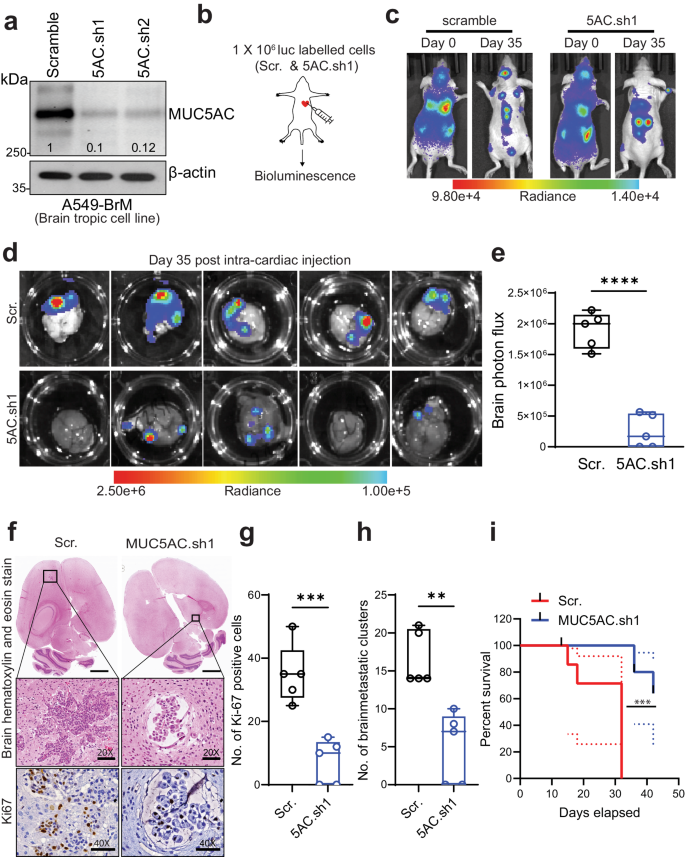
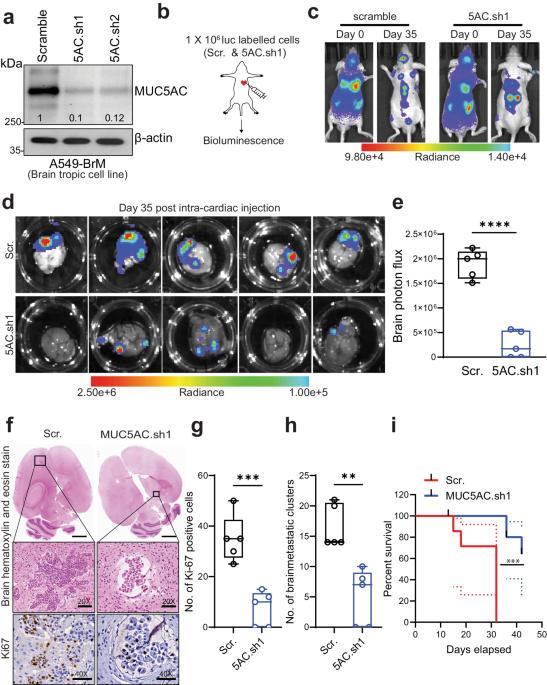
Non-small cell lung carcinoma (NSCLC) exhibits a heightened propensity for brain metastasis, posing a significant clinical challenge. Mucin 5ac (MUC5AC) plays a pivotal role in the development of lung adenocarcinoma (LUAD); however, its role in causing brain metastases remains unknown. In this study, we aimed to investigate the contribution of MUC5AC to brain metastasis in patients with LUAD utilizing various brain metastasis models. Our findings revealed a substantial increase in the MUC5AC level in LUAD brain metastases (LUAD-BrM) samples and brain-tropic cell lines compared to primary samples or parental control cell lines. Intriguingly, depletion of MUC5AC in brain-tropic cells led to significant reductions in intracranial metastasis and tumor growth, and improved survival following intracardiac injection, in contrast to the observations in the control groups. Proteomic analysis revealed that mechanistically, MUC5AC depletion resulted in decreased expression of metastasis-associated molecules. There were increases in epithelial-to-mesenchymal transition, tumor invasiveness, and metastasis phenotypes in tumors with high MUC5AC expression. Furthermore, immunoprecipitation and proteomic analysis revealed a novel interaction of MUC5AC with Annexin A2 (ANXA2), which activated downstream matrix metalloproteases and facilitated extracellular matrix degradation to promote metastasis. Disrupting MUC5AC-ANXA2 signaling with a peptide inhibitor effectively abrogated the metastatic process. Additionally, treatment of tumor cells with an astrocyte-conditioned medium or the chemokine CCL2 resulted in upregulation of MUC5AC expression and enhanced brain colonization. In summary, our study demonstrates that the MUC5AC/ANXA2 signaling axis promotes brain metastasis, suggesting a potential therapeutic paradigm for LUAD patients with high MUC5AC expression. Lung cancer frequently moves to the brain, but why is unclear. Scientists have found that a protein, MUC5AC, is crucial in this. The research, led by Sanjib Chaudhary and team, discovered that MUC5AC works with another protein, ANXA2, to help lung cancer cells move to the brain. They also found that astrocytes (a type of brain cell), release a substance that boosts the presence of MUC5AC in lung cancer cells. This research was a lab experiment using lung cancer cells and mice. They found that lowering MUC5AC in lung cancer cells greatly reduced their movement to the brain in mice. This suggests that focusing on MUC5AC could help stop lung cancer from moving to the brain. Future studies will need to confirm these results and look into possible treatments. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
剖析 MUC5AC/ANXA2 信号轴:对肺腺癌脑转移的影响
非小细胞肺癌(NSCLC)具有较高的脑转移倾向,给临床带来了巨大挑战。粘蛋白5ac(MUC5AC)在肺腺癌(LUAD)的发展过程中起着关键作用;然而,它在导致脑转移方面的作用仍然未知。在这项研究中,我们旨在利用各种脑转移模型研究 MUC5AC 对 LUAD 患者脑转移的贡献。我们的研究结果表明,与原发样本或亲代对照细胞系相比,LUAD 脑转移瘤(LUAD-BrM)样本和脑转移细胞系中的 MUC5AC 水平大幅升高。耐人寻味的是,在脑转移细胞中消耗MUC5AC可显著减少颅内转移和肿瘤生长,改善心内注射后的存活率,这与对照组的观察结果截然不同。蛋白质组学分析表明,从机理上讲,MUC5AC耗竭导致转移相关分子的表达减少。在MUC5AC高表达的肿瘤中,上皮细胞向间质转化、肿瘤侵袭性和转移表型均有所增加。此外,免疫沉淀和蛋白质组分析揭示了MUC5AC与Annexin A2(ANXA2)的新型相互作用,这种相互作用激活下游基质金属蛋白酶,促进细胞外基质降解,从而促进转移。用一种多肽抑制剂破坏MUC5AC-ANXA2信号转导可有效抑制转移过程。此外,用星形胶质细胞条件培养基或趋化因子CCL2处理肿瘤细胞会导致MUC5AC表达上调并增强脑定植。总之,我们的研究表明,MUC5AC/ANXA2 信号轴促进了脑转移,为 MUC5AC 高表达的 LUAD 患者提供了一种潜在的治疗模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


