An efficient, step-economical synthesis of β-carboline tethered imidazopyrido[3,4-b]indoles from acetals†
IF 2.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An efficient one-pot pseudo three component cascade annulation reaction has been devised to generate the fluorescent β-carboline tethered imidazopyrido[3,4-b]indole derivatives. These scaffolds were afforded in high yields via one-pot cascade reaction of diversified β-carboline acetals with NH4Cl through the formation of three C–N bonds in a single operation. The current protocol is step-economical and offers high atom-economy, short reaction time and high structural diversity with excellent yields (57–90%). The synthesized compounds displayed promising photophysical properties and delivered luminescence quantum yield (ΦF) up to 55%.
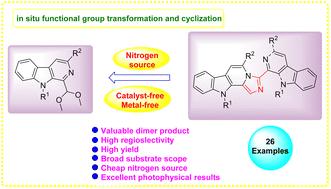
以乙缩醛为原料高效、分步经济地合成 β-咔啉系咪唑并吡啶并[3,4-b]吲哚
我们设计了一种高效的单锅伪三组分级联环化反应,生成了荧光 β-咔啉系咪唑并吡啶并[3,4-b]吲哚衍生物。通过在一次操作中形成三个 C-N 键,使多种 β-咔啉乙醛与 NH4Cl 发生一锅级联反应,从而以高产率获得了这些支架。目前的方案步骤经济,原子经济性高,反应时间短,结构多样性高,收率极佳(57-90%)。合成的化合物显示出良好的光物理特性,发光量子产率(ΦF)高达 55%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

New Journal of Chemistry
化学-化学综合
CiteScore
5.30
自引率
6.10%
发文量
1832
审稿时长
2 months
期刊介绍:
A journal for new directions in chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


