Analytical Interference in Chemiluminescence Assay–Measured Angiotensin I, Angiotensin II, Aldosterone, and Renin
Abstract
Background
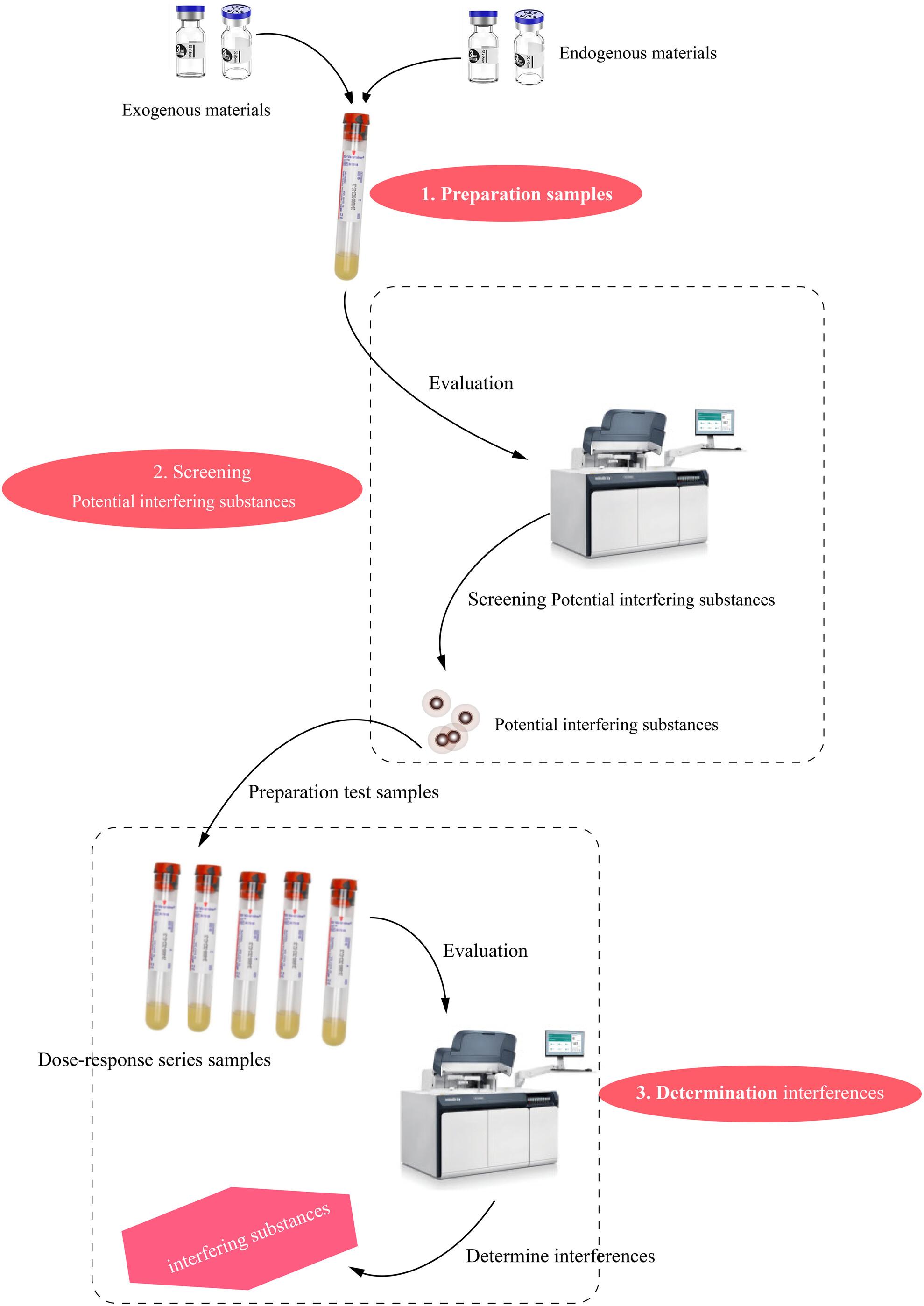
The interference can be a significant source of laboratory errors with the potential to cause immunoassay results to drift. Therefore, we evaluated the interference in various endogenous and exogenous substances on immunoassay for angiotensin I (Ang I), angiotensin II (Ang II), aldosterone, and renin in vitro.
Methods
Ten endogenous and eight exogenous substances were evaluated at supraphysiologic or supratherapeutic plasma levels using the screening study to identify potential interfering substances. Subsequently, potential interfering substances were further tested within maximum pathological or therapeutic plasma concentration ranges using the dose–response study to determine whether the interference has a significant bias. According to preset acceptance criteria, the interference in potential interfering substances for Ang I, Ang II, and renin and aldosterone assays was determined.
Results
Six potential interfering substances for Ang I immunoassays were identified, namely valsartan, nifedipine, spironolactone, cholesterol, hemoglobin, and triglyceride. Meanwhile, ethanol, nifedipine, spironolactone, heparin sodium, warfarin, hemoglobin, uric acid, cholesterol, and triglyceride appeared to have potential interference in the Ang II assay. Three identified as possible interferents for aldosterone immunoassays were glucose, valsartan, and spironolactone. Moreover, warfarin, valsartan, spironolactone, uric acid, cholesterol, bilirubin unconjugated, triglyceride, and hemoglobin were potential interfering substances for renin immunoassays. However, only spironolactone of these potential interfering substances exceeded preset mean bias limits (less than ±10.0%) in aldosterone immunoassays.
Conclusion
Exogenous spironolactone caused clinically significant interference in aldosterone immunoassays. Moreover, the interference in other substances was acceptable in Ang I, Ang II, and renin and aldosterone immunoassays.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


