Nano-sensitizer with self-amplified drug release and hypoxia normalization properties potentiates efficient chemoradiotherapy of pancreatic cancer
Abstract
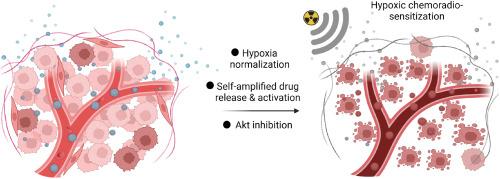
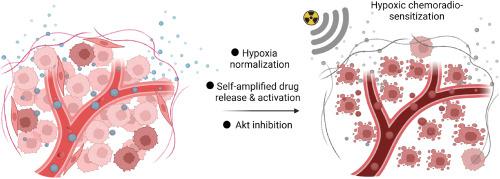
The hypoxic nature of pancreatic cancer, one of the most lethal malignancies worldwide, significantly impedes the effectiveness of chemoradiotherapy. Although the development of oxygen carriers and hypoxic sensitizers has shown promise in overcoming tumor hypoxia. The heterogeneity of hypoxia—primarily caused by limited oxygen penetration—has posed challenges. In this study, we designed a hypoxia-responsive nano-sensitizer by co-loading tirapazamine (TPZ), KP372-1, and MK-2206 in a metronidazole-modified polymeric vesicle. This nano-sensitizer relies on efficient endogenous NAD(P)H quinone oxidoreductase 1-mediated redox cycling induced by KP372-1, continuously consuming periphery oxygen and achieving evenly distributed hypoxia. Consequently, the normalized tumor microenvironment facilitates the self-amplified release and activation of TPZ without requiring deep penetration. The activated TPZ and metronidazole further sensitize radiotherapy, significantly reducing the radiation dose needed for extensive cell damage. Additionally, the coloaded MK-2206 complements inhibition of therapeutic resistance caused by Akt activation, synergistically enhancing the hypoxic chemoradiotherapy. This successful hypoxia normalization strategy not only overcomes hypoxia resistance in pancreatic cancer but also provides a potential universal approach to sensitize hypoxic tumor chemoradiotherapy by reshaping the hypoxic distribution.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


