Biomimetic Nanoparticles Carrying a Repolarization Agent of Tumor-Associated Macrophages for Remodeling of the Inflammatory Microenvironment Following Photothermal Therapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 53
Abstract
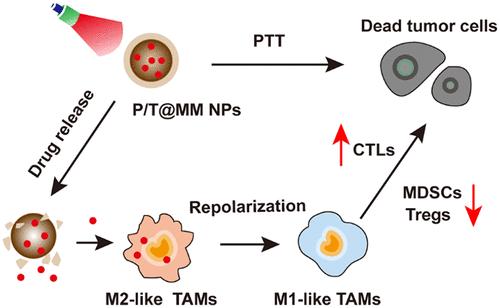
The complete regression of residual tumors after photothermal therapy (PTT) depends on the activation and recognition of the immune system. However, the inevitable local inflammation after PTT in residual tumor recruits abundant abnormal immune cells, especially the tumor-associated macrophages (TAMs) which further promote immune escape and survival of the remaining tumor cells, resulting in the tumor recurrence and progression. To solve this problem, herein we explored biomimetic nanoparticles carrying repolarization agent of TAMs to remodel the post-PTT inflammatory microenvironment. The polydopamine nanoparticles were used simultaneously as photothermal transduction agents to ablate tumor cells and the delivery vehicles for TMP195 which can repolarize the M2-like TAMs into an antitumor phenotype. In addition, a biomimetic decoration of macrophage membrane coating was designed to endow nanoparticles the ability to actively target the tumor site after PTT mediated by inflammation-mediated chemotaxis. In the breast tumor model, these biomimetic nanoparticles with immune-modulating ability significantly elevated the levels of M1-like TAMs, ultimately resulting in a tumor-elimination rate of 60%, increased from 10% after PTT. This synergistic treatment strategy of PTT and TAMs repolarization provides a promising approach to address the deteriorated tumor microenvironment after PTT and proposes a more effective way for combinational treatment option in clinic.
携带肿瘤相关巨噬细胞复极化剂的仿生纳米颗粒用于光热治疗后炎症微环境的重塑
光热治疗(PTT)后残余肿瘤的完全消退依赖于免疫系统的激活和识别。然而,残余肿瘤PTT后不可避免的局部炎症会招募大量异常免疫细胞,尤其是肿瘤相关巨噬细胞(tumor-associated macrophages, tam),进一步促进残余肿瘤细胞的免疫逃逸和存活,导致肿瘤复发和进展。为了解决这一问题,我们探索了携带tam复极化剂的仿生纳米颗粒来重塑ptt后的炎症微环境。聚多巴胺纳米颗粒同时作为光热转导剂消融肿瘤细胞和TMP195的递送载体,TMP195可以将m2样tam重新极化为抗肿瘤表型。此外,设计了巨噬细胞膜涂层的仿生装饰,使纳米颗粒能够在炎症介导的趋化性介导的PTT后主动靶向肿瘤部位。在乳腺肿瘤模型中,这些具有免疫调节能力的仿生纳米颗粒显著提高了m1样tam的水平,最终使肿瘤消除率从PTT后的10%提高到60%。这种PTT与tam复极化的协同治疗策略为解决PTT后肿瘤微环境恶化提供了一条有希望的途径,并为临床的联合治疗选择提供了更有效的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


