Targeting a lineage-specific PI3Kɣ–Akt signaling module in acute myeloid leukemia using a heterobifunctional degrader molecule
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
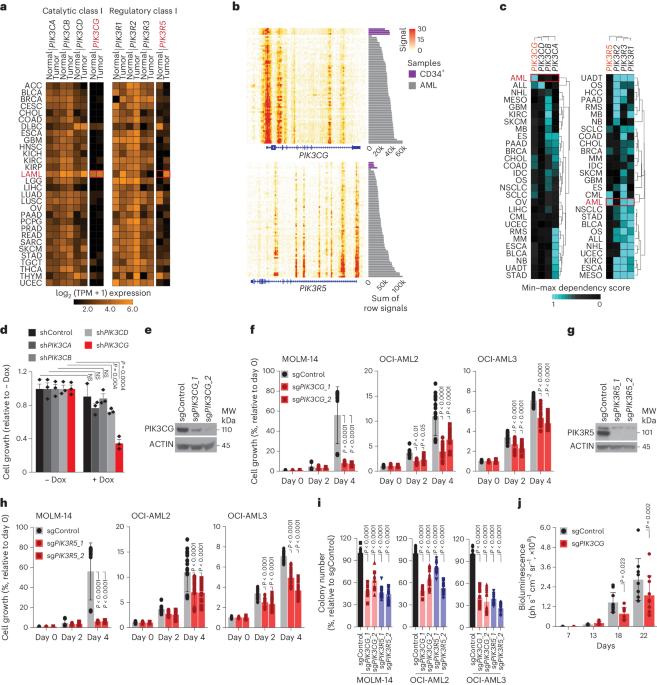
Dose-limiting toxicity poses a major limitation to the clinical utility of targeted cancer therapies, often arising from target engagement in nonmalignant tissues. This obstacle can be minimized by targeting cancer dependencies driven by proteins with tissue-restricted and/or tumor-restricted expression. In line with another recent report, we show here that, in acute myeloid leukemia (AML), suppression of the myeloid-restricted PIK3CG/p110γ–PIK3R5/p101 axis inhibits protein kinase B/Akt signaling and compromises AML cell fitness. Furthermore, silencing the genes encoding PIK3CG/p110γ or PIK3R5/p101 sensitizes AML cells to established AML therapies. Importantly, we find that existing small-molecule inhibitors against PIK3CG are insufficient to achieve a sustained long-term antileukemic effect. To address this concern, we developed a proteolysis-targeting chimera (PROTAC) heterobifunctional molecule that specifically degrades PIK3CG and potently suppresses AML progression alone and in combination with venetoclax in human AML cell lines, primary samples from patients with AML and syngeneic mouse models. Puissant and colleagues identify a myeloid-restricted PIK3CG/p110γ–PIK3R5/p101 axis as a therapeutic vulnerability in acute myeloid leukemia and develop a proteolysis-targeting chimera to potently degrade PIK3CG and suppress AML progression.
利用异功能降解分子靶向急性髓性白血病中的特异性 PI3Kɣ-Akt 信号模块。
剂量限制性毒性是癌症靶向疗法临床应用的一个主要限制因素,通常是由非恶性组织中的靶点参与引起的。通过靶向受组织限制和/或肿瘤限制表达的蛋白质所驱动的癌症依赖性,可以最大限度地减少这一障碍。与最近的另一篇报道一致,我们在此表明,在急性髓性白血病(AML)中,抑制髓限制性 PIK3CG/p110γ-PIK3R5/p101 轴可抑制蛋白激酶 B/Akt 信号转导,并损害 AML 细胞的健康。此外,沉默编码 PIK3CG/p110γ 或 PIK3R5/p101 的基因会使 AML 细胞对现有的 AML 疗法敏感。重要的是,我们发现现有的 PIK3CG 小分子抑制剂不足以实现长期持续的抗白血病效果。为了解决这个问题,我们开发了一种蛋白水解靶向嵌合体(PROTAC)异功能分子,它能特异性降解 PIK3CG,在人类急性髓细胞白血病细胞系、急性髓细胞白血病患者的原始样本和合成小鼠模型中单独或与 Venetoclax 联用都能有效抑制急性髓细胞白血病的进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


