Mitochondrial RelA empowers mtDNA G-quadruplex formation for hypoxia adaptation in cancer cells
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Mitochondrial DNA (mtDNA) G-quadruplexes (G4s) have important regulatory roles in energy metabolism, yet their specific functions and underlying regulatory mechanisms have not been delineated. Using a chemical-genetic screening strategy, we demonstrated that the JAK/STAT3 pathway is the primary regulatory mechanism governing mtDNA G4 dynamics in hypoxic cancer cells. Further proteomic analysis showed that activation of the JAK/STAT3 pathway facilitates the translocation of RelA, a member of the NF-κB family, to the mitochondria, where RelA binds to mtDNA G4s and promotes their folding, resulting in increased mtDNA instability, inhibited mtDNA transcription, and subsequent mitochondrial dysfunction. This binding event disrupts the equilibrium of energy metabolism, catalyzing a metabolic shift favoring glycolysis. Collectively, the results provide insights into a strategy employed by cancer cells to adapt to hypoxia through metabolic reprogramming.
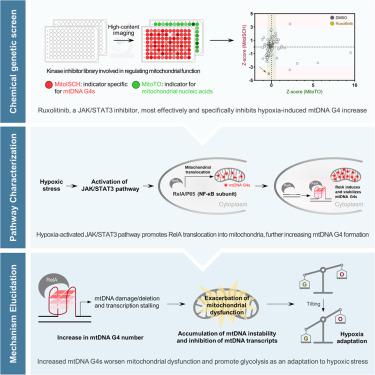
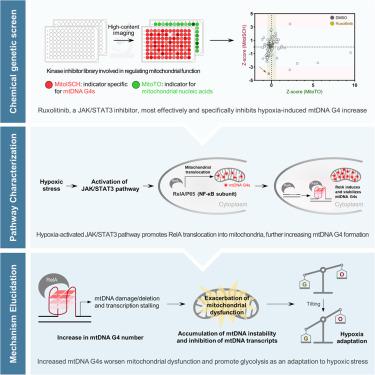
线粒体 RelA 促进了 mtDNA G-四联体的形成,使癌细胞适应缺氧环境
线粒体 DNA(mtDNA)G-四重链(G4s)在能量代谢中具有重要的调控作用,但其具体功能和潜在的调控机制尚未明确。利用化学遗传筛选策略,我们证明了 JAK/STAT3 通路是缺氧癌细胞中 mtDNA G4 动态的主要调控机制。进一步的蛋白质组分析表明,JAK/STAT3 通路的激活促进了 NF-κB 家族成员 RelA 向线粒体的转位,RelA 在线粒体中与 mtDNA G4 结合并促进其折叠,导致 mtDNA 不稳定性增加、mtDNA 转录受抑制以及随后的线粒体功能障碍。这一结合事件破坏了能量代谢的平衡,催化了有利于糖酵解的代谢转变。总之,这些结果让我们了解到癌细胞通过代谢重编程来适应缺氧的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


