Pro-CRISPR PcrIIC1-associated Cas9 system for enhanced bacterial immunity
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
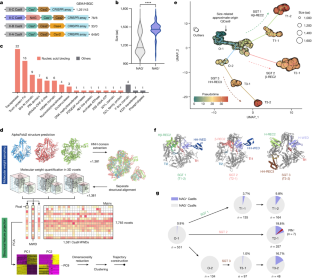
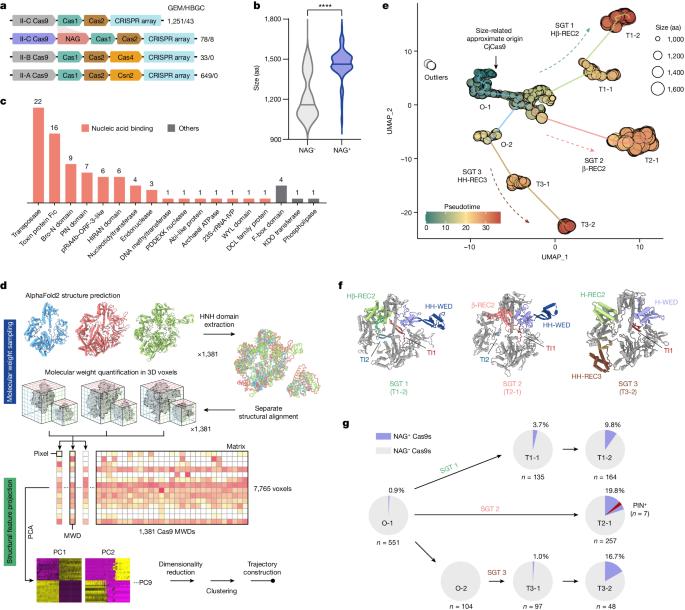
The CRISPR system is an adaptive immune system found in prokaryotes that defends host cells against the invasion of foreign DNA1. As part of the ongoing struggle between phages and the bacterial immune system, the CRISPR system has evolved into various types, each with distinct functionalities2. Type II Cas9 is the most extensively studied of these systems and has diverse subtypes. It remains uncertain whether members of this family can evolve additional mechanisms to counter viral invasions3,4. Here we identify 2,062 complete Cas9 loci, predict the structures of their associated proteins and reveal three structural growth trajectories for type II-C Cas9. We found that novel associated genes (NAGs) tended to be present within the loci of larger II-C Cas9s. Further investigation revealed that CbCas9 from Chryseobacterium species contains a novel β-REC2 domain, and forms a heterotetrameric complex with an NAG-encoded CRISPR–Cas-system-promoting (pro-CRISPR) protein of II-C Cas9 (PcrIIC1). The CbCas9–PcrIIC1 complex exhibits enhanced DNA binding and cleavage activity, broader compatibility for protospacer adjacent motif sequences, increased tolerance for mismatches and improved anti-phage immunity, compared with stand-alone CbCas9. Overall, our work sheds light on the diversity and ‘growth evolutionary’ trajectories of II-C Cas9 proteins at the structural level, and identifies many NAGs—such as PcrIIC1, which serves as a pro-CRISPR factor to enhance CRISPR-mediated immunity. Comprehensive analyses of Cas9 proteins shed light on the evolution of the CRISPR–Cas9 system, and identify a pro-CRISPR accessory protein in bacteria that boosts CRISPR-mediated immunity by enhancing the DNA binding and cleavage activity of Cas9.
用于增强细菌免疫力的 Pro-CRISPR PcrIIC1-associated Cas9 系统。
CRISPR 系统是原核生物中的一种适应性免疫系统,可抵御外来 DNA1 入侵宿主细胞。在噬菌体与细菌免疫系统的持续斗争中,CRISPR 系统进化成了多种类型,每种类型都具有不同的功能2。II 型 Cas9 是这些系统中研究最为广泛的一种,具有多种亚型。目前仍不确定该家族成员是否能进化出其他机制来对抗病毒入侵3,4。在这里,我们确定了 2,062 个完整的 Cas9 基因座,预测了其相关蛋白的结构,并揭示了 II-C 型 Cas9 的三种结构生长轨迹。我们发现,新的相关基因(NAG)往往存在于较大的 II-C Cas9 的基因座中。进一步研究发现,来自 Chryseobacterium 物种的 CbCas9 含有一个新的β-REC2 结构域,并与 NAG 编码的 II-C Cas9 的 CRISPR-Cas 系统促进蛋白(pro-CRISPR)(PcrIIC1)形成异源四聚体复合物。与独立的 CbCas9 相比,CbCas9-PcrIIC1 复合物表现出更强的 DNA 结合力和切割活性、对原间隔相邻基序更广泛的兼容性、对错配更强的耐受力以及更好的抗噬菌体免疫力。总之,我们的研究揭示了 II-C Cas9 蛋白在结构水平上的多样性和 "生长进化 "轨迹,并发现了许多 NAGs,如 PcrIIC1,它是一种支持 CRISPR 的因子,可增强 CRISPR 介导的免疫力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


