A unifying model for membrane protein biogenesis
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
α-Helical integral membrane proteins comprise approximately 25% of the proteome in all organisms. The membrane proteome is highly diverse, varying in the number, topology, spacing and properties of transmembrane domains. This diversity imposes different constraints on the insertion of different regions of a membrane protein into the lipid bilayer. Here, we present a cohesive framework to explain membrane protein biogenesis, in which different parts of a nascent substrate are triaged between Oxa1 and SecY family members for insertion. In this model, Oxa1 family proteins insert transmembrane domains flanked by short translocated segments, whereas the SecY channel is required for insertion of transmembrane domains flanked by long translocated segments. Our unifying model rationalizes evolutionary, genetic, biochemical and structural data across organisms and provides a foundation for future mechanistic studies of membrane protein biogenesis. In this Perspective, the authors propose a framework to explain membrane protein biogenesis, wherein different parts of a nascent substrate are triaged between Oxa1 and SecY family members for insertion.
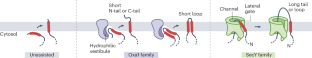
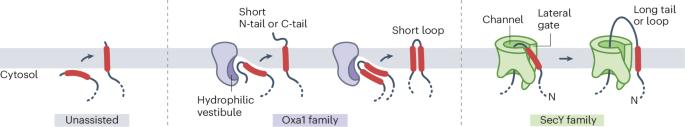
膜蛋白生物生成的统一模型
α-螺旋整体膜蛋白约占所有生物体蛋白质组的 25%。膜蛋白质组种类繁多,跨膜结构域的数量、拓扑结构、间距和特性各不相同。这种多样性对膜蛋白的不同区域插入脂质双分子层施加了不同的限制。在这里,我们提出了一个解释膜蛋白生物发生的内聚框架,在这个框架中,新生底物的不同部分在 Oxa1 和 SecY 家族成员之间进行分流,以便插入。在这个模型中,Oxa1 家族蛋白插入侧翼为短转位片段的跨膜结构域,而 SecY 通道则需要插入侧翼为长转位片段的跨膜结构域。我们的统一模型合理地整合了各种生物的进化、遗传、生化和结构数据,为未来膜蛋白生物发生的机理研究奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


