Virus-like structures for combination antigen protein mRNA vaccination
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
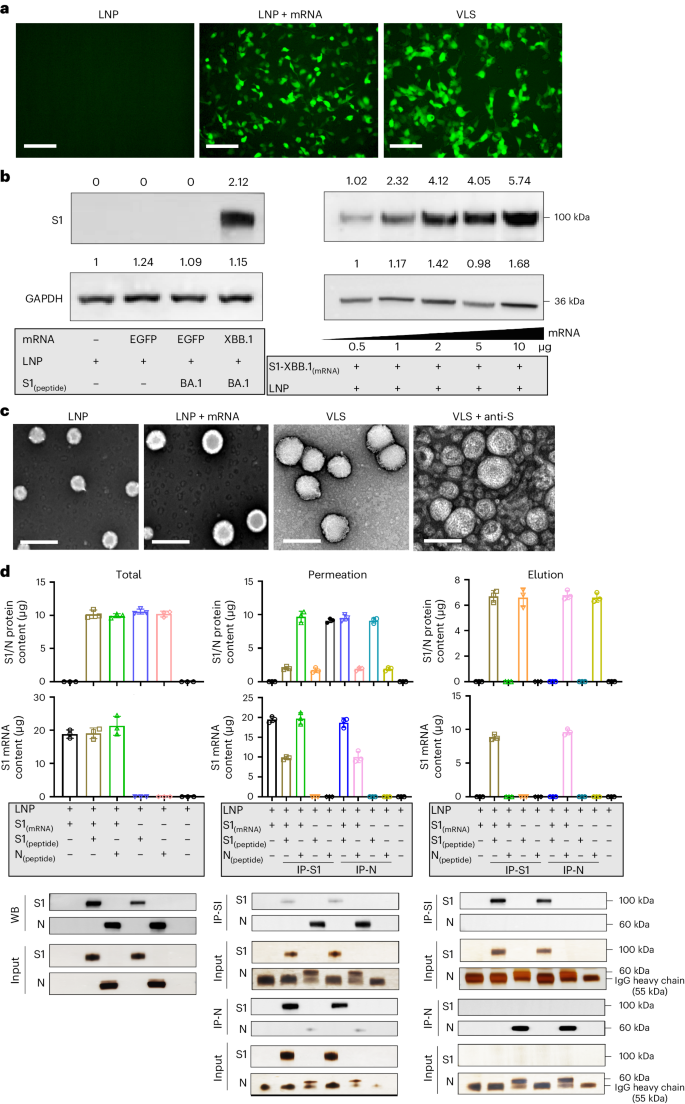
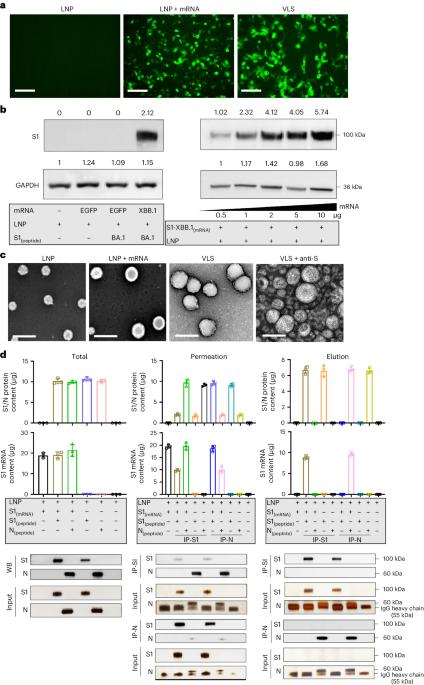
Improved vaccination requires better delivery of antigens and activation of the natural immune response. Here we report a lipid nanoparticle system with the capacity to carry antigens, including mRNA and proteins, which is formed into a virus-like structure by surface decoration with spike proteins, demonstrating application against SARS-CoV-2 variants. The strategy uses S1 protein from Omicron BA.1 on the surface to deliver mRNA of S1 protein from XBB.1. The virus-like particle enables specific augmentation of mRNAs expressed in human respiratory epithelial cells and macrophages via the interaction the surface S1 protein with ACE2 or DC-SIGN receptors. Activation of macrophages and dendritic cells is demonstrated by the same receptor binding. The combination of protein and mRNA increases the antibody response in BALB/c mice compared with mRNA and protein vaccines alone. Our exploration of the mechanism of this robust immunity suggests it might involve cross-presentation to diverse subsets of dendritic cells ranging from activated innate immune signals to adaptive immune signals. This paper presents a virus-like lipid nanoparticle decorated with spike proteins capable of carrying antigens, including mRNA and proteins, for vaccination against SARS-CoV-2 variants.
用于组合抗原蛋白 mRNA 疫苗的病毒样结构
改进疫苗接种需要更好地传递抗原和激活天然免疫反应。在此,我们报告了一种具有携带抗原(包括 mRNA 和蛋白质)能力的脂质纳米粒子系统,该系统通过表面装饰尖峰蛋白形成类似病毒的结构,并在抗 SARS-CoV-2 变体方面得到了应用。该策略利用表面的 Omicron BA.1 S1 蛋白来传递 XBB.1 S1 蛋白的 mRNA。病毒样颗粒通过表面 S1 蛋白与 ACE2 或 DC-SIGN 受体的相互作用,特异性地增强了在人类呼吸道上皮细胞和巨噬细胞中表达的 mRNA。巨噬细胞和树突状细胞的活化也是通过相同的受体结合实现的。与单独使用 mRNA 和蛋白疫苗相比,蛋白和 mRNA 的结合可提高 BALB/c 小鼠的抗体反应。我们对这种强效免疫机制的探索表明,它可能涉及到从激活的先天性免疫信号到适应性免疫信号等不同树突状细胞亚群的交叉呈递。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


