EDTA functionalized pine needle biochar (EDTA@BC); a valorized bio-material for removal of Ni(II) from aqueous solution
Abstract
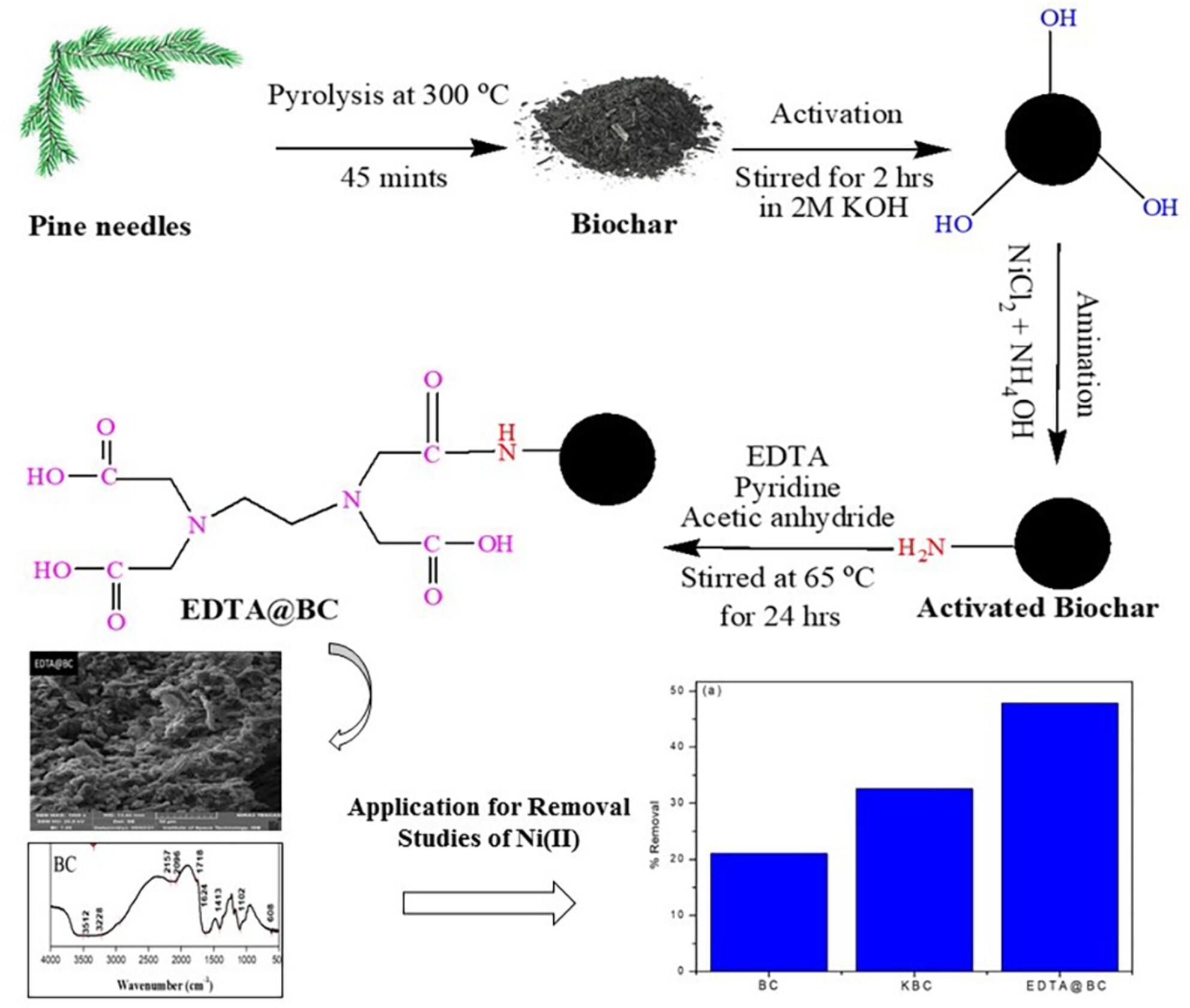
The preparation of ethylenediaminetetraacetic acid (EDTA) functionalized pine needles biochar (EDTA@BC) as a low-cost active adsorbent and its effectiveness in removing Ni(II) from aqueous solution at various conditions is reported in this paper. First, alkali activation was selected to render the pine needle biochar with an excellent porous structure and increased concentration of hydroxyl groups to facilitate grafting. Subsequently, a simple method was utilized to graft EDTA onto the biochar. The prepared EDTA@BC was characterized by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy dispersive x-ray spectrometry (EDX). Batch adsorption studies were conducted to assess the impact of various parameters such as solution pH, adsorbent dosage, adsorbate volume, and shaking time on the removal efficiency of Ni(II). At pH 6, 100 mg dosage, 4 mL of adsorbate volume, and 10 min of shaking time, the maximum removal efficiency of Ni(II) was observed to be 89%. EDTA@BC showed reasonable sorption performance still after the third cycle of regeneration. The effect of interfering ions such as Pb, Cr, Cu, and Hg was evaluated, resulting a decrease of 69%, 78%, 76%, and 68%, respectively, in its sorption capacity. The Langmuir model provided a better fit for Ni(II) in the concentration range of 0.1–2000 ppm under optimized conditions, with qmax of 46.69 ± 1.031 mg/g and KL of 0.001, compared with the Freundlich isotherm, which yielded n = 0.234 and χ2 = 2.7899, Temkin isotherm (R2 = 0.9520), and Redlich-Peterson isotherm (R2 = 0.9725). The removal of Ni(II) by EDTA@BC was found to be the pseudo-second-order kinetics. Thermodynamic studies indicated adsorption process to be endothermic and nonspontaneous. Hence, a sustainable valorized bio-material (EDTA@BC) is prepared having better sorption efficiency of Ni(II) from aqueous solution with possible wide applicability.
Research Highlights
- New EDTA functionalized indigenous pine needles biochar (EDTA@BC) was prepared.
- This low-cost active adsorbent found effective in removing Ni(II) from aqueous solution.
- FTIR, SEM, and EDX proved synthesis and uptake of Ni(II) from aqueous solution.
- Ni(II) removal, regeneration, interfering and adsorption studies were performed by UV–Vis spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


