Enhanced heavy metal adsorption capacity of surface-functionalized Philippine natural zeolite in simulated wastewater
Abstract
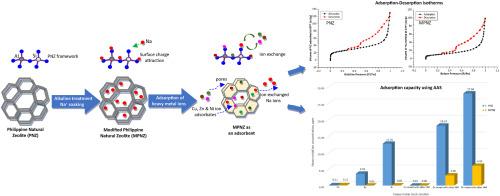
The adsorption efficiency of surface-functionalized Philippine natural zeolite (PNZ) for heavy metal uptake from single and mixed metal ion-simulated wastewater solution is reported in this work. Atomic adsorption spectroscopy (AAS) findings revealed that NaCl-modified PNZ (MPNZ) exhibited the highest zinc ion adsorption of 99.96 % while PNZ yielded an adsorption of 95.61 %. The adsorption isotherms of raw PNZ and MPNZ both show similar shapes that reflect Type IV adsorption-desorption isotherms with Type H2 hysteresis loop which can be observed for micro-mesoporous materials (pore containing 2–50 nm). An increase in PNZ pore size from 10.11 nm to 13.36 nm for NaCl-PNZ and 15.59 for NaOH-PNZ is observed after alkaline treatment. EDS confirmed the decrease in Si/Al ratio from 4.02 to 3.76, indicative of possible higher negative charge in the PNZ framework which is favorable for an enhanced Coulombic or electrostatic interaction with the cationic heavy metals being detected in this study. MPNZ demonstrated an adsorption capacity of 99.96 % for copper in mixed-ion solution while 88.49 % and 86.67 % were obtained for zinc and nickel, respectively. These values are higher compared to PNZ having only 32.21 % uptake for zinc and 38.00 % for nickel. A hierarchy of the average metal adsorption capacity showed the order: copper > nickel > zinc. Rapid adsorption at the first hour of the adsorption reaction was attained in all solutions while samples with pH 9 exhibited the highest Ni2+ and Zn2+ percent removal. Moreover, the increase in initial solution concentration led to lower adsorption efficiency and the maximum uptake was attained at 100 ppm. The equilibrium data of adsorption and mechanism were suitably described by Langmuir isotherm model. With these, surface functionalization of PNZ has further enhanced its cationic adsorption capacity on heavy metals in both single and mixed-ion solution having promising potential for wastewater remediation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


