Heart failure promotes multimorbidity through innate immune memory
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
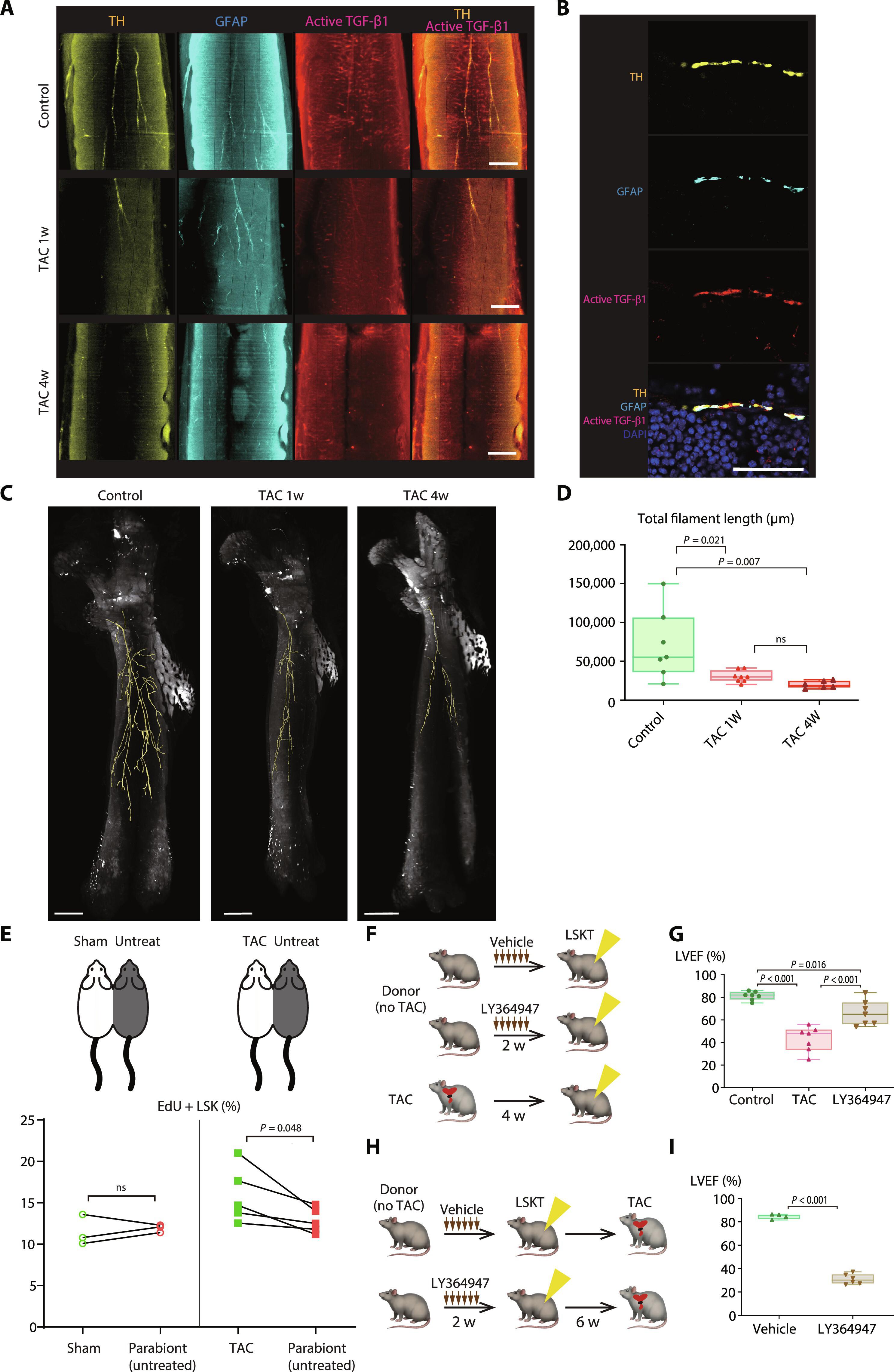
Patients with heart failure (HF) often experience repeated acute decompensation and develop comorbidities such as chronic kidney disease and frailty syndrome. Although this suggests pathological interaction among comorbidities, the mechanisms linking them are poorly understood. Here, we identified alterations in hematopoietic stem cells (HSCs) as a critical driver of recurrent HF and associated comorbidities. Bone marrow transplantation from HF-experienced mice resulted in spontaneous cardiac dysfunction and fibrosis in recipient mice, as well as increased vulnerability to kidney and skeletal muscle insults. HF enhanced the capacity of HSCs to generate proinflammatory macrophages. In HF mice, global chromatin accessibility analysis and single-cell RNA-seq showed that transforming growth factor–β (TGF-β) signaling was suppressed in HSCs, which corresponded with repressed sympathetic nervous activity in bone marrow. Transplantation of bone marrow from mice in which TGF-β signaling was inhibited similarly exacerbated cardiac dysfunction. Collectively, these results suggest that cardiac stress modulates the epigenome of HSCs, which in turn alters their capacity to generate cardiac macrophage subpopulations. This change in HSCs may be a common driver of repeated HF events and comorbidity by serving as a key carrier of “stress memory.”
心力衰竭通过先天性免疫记忆促进多病共存。
心力衰竭(HF)患者通常会反复出现急性失代偿,并发展为慢性肾病和虚弱综合征等合并症。虽然这表明合并症之间存在病理相互作用,但对它们之间的关联机制却知之甚少。在这里,我们发现造血干细胞(HSCs)的改变是复发性高血压和相关合并症的关键驱动因素。从有高房颤症经历的小鼠身上移植骨髓会导致受体小鼠自发性心脏功能障碍和纤维化,并增加对肾脏和骨骼肌损伤的脆弱性。高房血症增强了造血干细胞生成促炎巨噬细胞的能力。在高频小鼠中,全染色质可及性分析和单细胞RNA-seq显示,造血干细胞中的转化生长因子-β(TGF-β)信号传导受到抑制,这与骨髓中交感神经活动受到抑制相对应。移植 TGF-β 信号被抑制的小鼠骨髓同样会加重心脏功能障碍。总之,这些结果表明,心脏应激会改变造血干细胞的表观基因组,进而改变其生成心脏巨噬细胞亚群的能力。造血干细胞的这种变化可能是 "应激记忆 "的关键载体,是反复高频事件和合并症的共同驱动因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


