Tead4 and Tfap2c generate bipotency and a bistable switch in totipotent embryos to promote robust lineage diversification
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
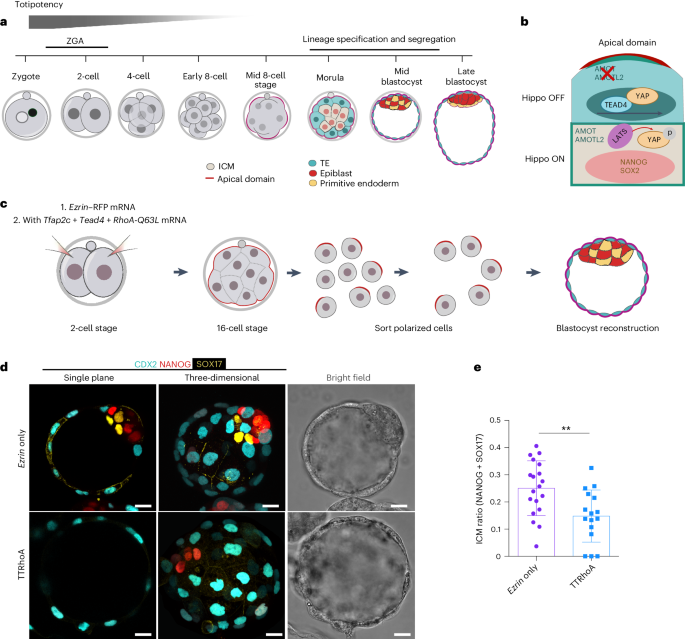
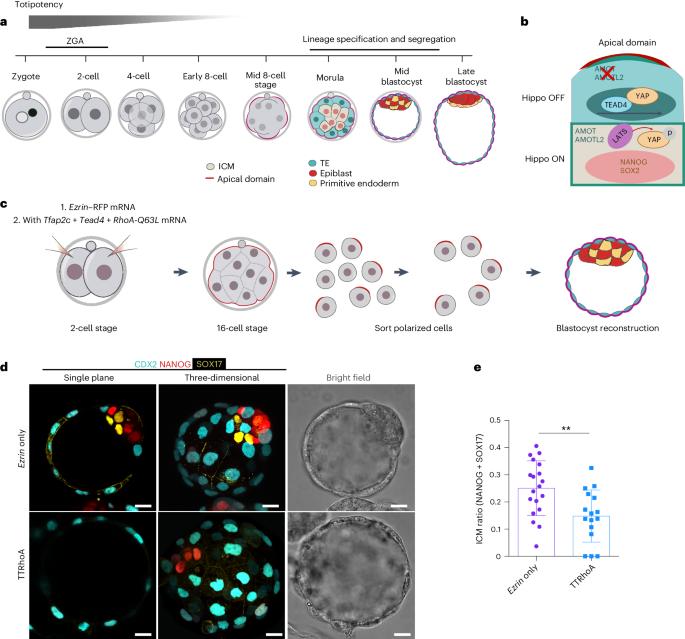
The mouse and human embryo gradually loses totipotency before diversifying into the inner cell mass (ICM, future organism) and trophectoderm (TE, future placenta). The transcription factors TFAP2C and TEAD4 with activated RHOA accelerate embryo polarization. Here we show that these factors also accelerate the loss of totipotency. TFAP2C and TEAD4 paradoxically promote and inhibit Hippo signaling before lineage diversification: they drive expression of multiple Hippo regulators while also promoting apical domain formation, which inactivates Hippo. Each factor activates TE specifiers in bipotent cells, while TFAP2C also activates specifiers of the ICM fate. Asymmetric segregation of the apical domain reconciles the opposing regulation of Hippo signaling into Hippo OFF and the TE fate, or Hippo ON and the ICM fate. We propose that the bistable switch established by TFAP2C and TEAD4 is exploited to trigger robust lineage diversification in the developing embryo. Here the authors identify the transcription factors TFAP2C and TEAD4 as a bistable switch that reconciles into Hippo ON and OFF states, establishing a composite state at the eight-cell stage and critically regulating lineage diversification.
Tead4 和 Tfap2c 在全能胚胎中产生双能性和双稳态开关,促进稳健的血统多样化
小鼠和人类胚胎在分化为内细胞团(ICM,未来的生物体)和滋养外胚层(TE,未来的胎盘)之前,会逐渐丧失全能性。转录因子 TFAP2C 和 TEAD4 与活化的 RHOA 可加速胚胎极化。在这里,我们发现这些因子也会加速全能性的丧失。TFAP2C和TEAD4在品系分化之前既促进又抑制Hippo信号:它们在驱动多种Hippo调节因子表达的同时也促进顶端结构域的形成,从而使Hippo失活。每个因子都能激活双能细胞中的TE特异体,而TFAP2C也能激活ICM命运的特异体。顶端结构域的非对称分离调和了Hippo信号的相反调控,即Hippo OFF和TE命运,或Hippo ON和ICM命运。我们认为,TFAP2C和TEAD4建立的双稳态开关被用来触发发育中胚胎的稳健品系分化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


