Systematic evaluation of single-cell RNA-seq analyses performance based on long-read sequencing platforms
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
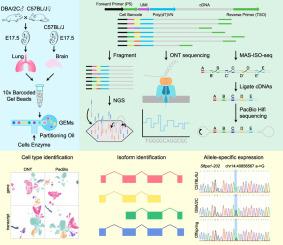
The rapid development of next-generation sequencing (NGS)-based single-cell RNA sequencing (scRNA-seq) allows for detecting and quantifying gene expression in a high-throughput manner, providing a powerful tool for comprehensively understanding cellular function in various biological processes. However, the NGS-based scRNA-seq only quantifies gene expression and cannot reveal the exact transcript structures (isoforms) of each gene due to the limited read length. On the other hand, the long read length of third-generation sequencing (TGS) technologies, including Oxford Nanopore Technologies (ONT) and Pacific Biosciences (PacBio), enable direct reading of intact cDNA molecules.
Objectives
Both ONT and PacBio have been used in conjunction with scRNA-seq, but their performance in single-cell analyses has not been systematically evaluated.
Methods
To address this, we generated ONT and PacBio data from the same single-cell cDNA libraries containing different amount of cells.
Results
Using NGS as a control, we assessed the performance of each platform in cell type identification. Additionally, the reliability in identifying novel isoforms and allele-specific gene/isoform expression by both platforms was verified, providing a systematic evaluation to design the sequencing strategies in single-cell transcriptome studies.
Conclusion
Beyond gene expression analysis, which the NGS-based scRNA-seq only affords, TGS-based scRNA-seq achieved gene splicing analyses, identifying novel isoforms. Attribute to higher sequencing quality of PacBio, it outperforms ONT in accuracy of novel transcripts identification and allele-specific gene/isoform expression.

基于长线程测序平台的单细胞RNA-seq分析性能系统评估
导言:基于下一代测序(NGS)的单细胞 RNA 测序(scRNA-seq)技术发展迅速,可以高通量地检测和量化基因表达,为全面了解各种生物过程中的细胞功能提供了强有力的工具。然而,基于 NGS 的 scRNA-seq 只能量化基因表达,由于读长有限,无法揭示每个基因的确切转录本结构(异构体)。另一方面,第三代测序(TGS)技术(包括牛津纳米孔技术公司(ONT)和太平洋生物科学公司(PacBio))的长读取长度可以直接读取完整的 cDNA 分子:目的:ONT 和 PacBio 已与 scRNA-seq 结合使用,但它们在单细胞分析中的性能尚未得到系统评估:为了解决这个问题,我们从含有不同数量细胞的相同单细胞 cDNA 文库中生成了 ONT 和 PacBio 数据:结果:以 NGS 作为对照,我们评估了每个平台在细胞类型鉴定方面的性能。此外,我们还验证了两种平台在鉴定新型同工酶和等位基因特异性基因/同工酶表达方面的可靠性,为设计单细胞转录组研究中的测序策略提供了系统性评估:结论:除了基于 NGS 的 scRNA-seq 只能进行基因表达分析外,基于 TGS 的 scRNA-seq 还能进行基因剪接分析,识别新的同工酶体。由于 PacBio 的测序质量更高,它在鉴定新转录本和等位基因特异性基因/同工酶表达的准确性方面优于 ONT。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


