Critical outcomes to be included in the Core Outcome Set for nutritional intervention studies in older adults with malnutrition or at risk of malnutrition: a modified Delphi Study
IF 3.6
3区 医学
Q2 NUTRITION & DIETETICS
引用次数: 0
Abstract
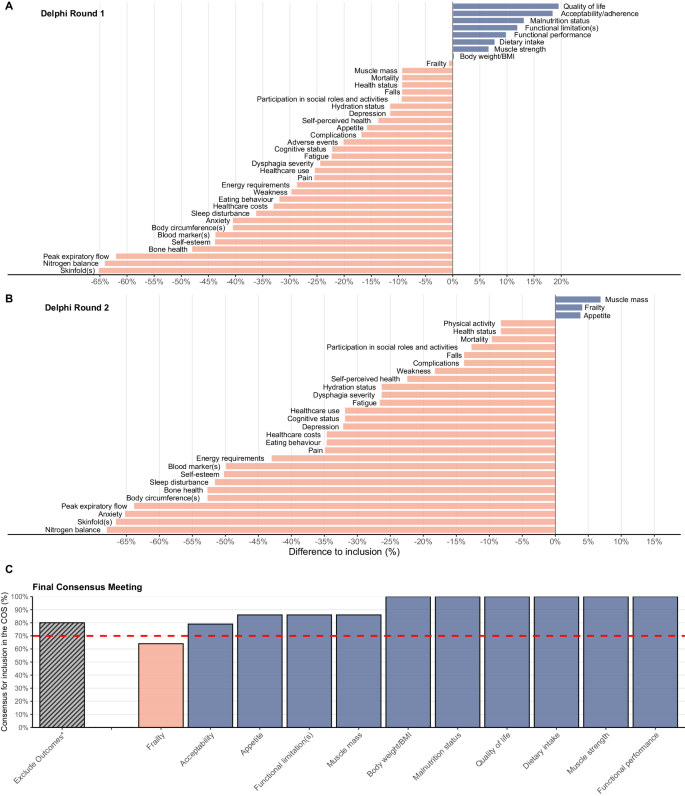
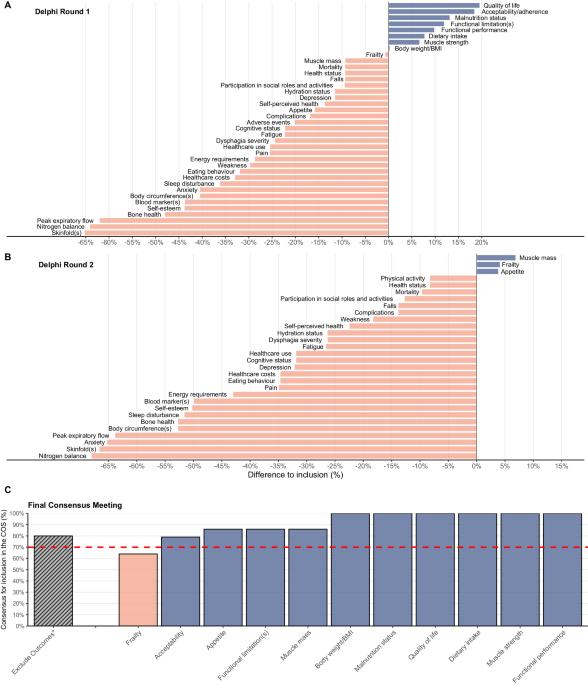
As part of the development of an agreed minimum set of outcomes or Core Outcome Set (COS) for future nutritional intervention trials in older adults with malnutrition or at risk of malnutrition, this work reports on the Delphi surveys and final consensus. Outcomes from a scoping review were incorporated into a two-round Delphi survey. Researchers and healthcare professionals experienced in malnutrition in older adults were invited to take part in an online survey to rate 38 selected outcomes on a nine-point Likert scale ranging from ‘not important’ to ‘critical’ for their setting (community, hospital, or long-term care). Consensus for inclusion was reached when ≥75% (or ≥60% if a patient-reported outcome) of the participants scored the outcome as ‘critical’ and <15% as ‘not important’. Resulting outcomes were voted for inclusion or exclusion in the COS in a final online consensus meeting. Ninety-three and 72 participants from diverse professional backgrounds and countries participated in the 1st and 2nd Delphi round, respectively. After both rounds eleven outcomes met the inclusion criteria, largely irrespective of setting. Fifteen participants, representing academia, health care, health policy, industry, and PPI, voted in a final online consensus meeting resulting in ten outcomes: malnutrition status, dietary intake, appetite, body weight or BMI, muscle strength, muscle mass, functional performance, functional limitations, quality of life, and acceptability of the intervention. Ten outcomes will form the COS which is intended to be used by the scientific community in all future nutritional intervention studies for older adults with malnutrition or at risk of malnutrition. The subsequent phase will establish the appropriate methods to measure these outcomes.
营养不良或有营养不良风险的老年人营养干预研究核心成果集应包括的关键成果:改良德尔菲研究。
导言:作为为未来针对营养不良或有营养不良风险的老年人的营养干预试验制定一套公认的最低结果或核心结果集(COS)的一部分,本研究报告了德尔菲调查和最终共识:方法:将范围审查的结果纳入两轮德尔菲调查。研究人员和对老年人营养不良问题有经验的医疗保健专业人员受邀参加在线调查,按照李克特九点量表(从 "不重要 "到 "关键 "不等)对所选的 38 项结果进行评分。当≥75%的参与者(或≥60%的患者报告结果)将结果评分为 "关键 "时,即达成了纳入共识:来自不同专业背景和国家的 93 名和 72 名参与者分别参加了第一轮和第二轮德尔菲讨论。经过这两轮讨论,有 11 项结果符合纳入标准,基本上与背景无关。代表学术界、医疗保健、卫生政策、工业和公共宣传机构的 15 位参与者在最终的在线共识会议上进行了投票,最终得出了 10 项结果:营养不良状况、饮食摄入量、食欲、体重或体重指数、肌肉力量、肌肉质量、功能表现、功能限制、生活质量和干预的可接受性:十项结果将构成 COS,供科学界用于今后所有针对营养不良或有营养不良风险的老年人的营养干预研究。下一阶段将确定衡量这些结果的适当方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.60
自引率
2.10%
发文量
189
审稿时长
3-6 weeks
期刊介绍:
The European Journal of Clinical Nutrition (EJCN) is an international, peer-reviewed journal covering all aspects of human and clinical nutrition. The journal welcomes original research, reviews, case reports and brief communications based on clinical, metabolic and epidemiological studies that describe methodologies, mechanisms, associations and benefits of nutritional interventions for clinical disease and health promotion.
Topics of interest include but are not limited to:
Nutrition and Health (including climate and ecological aspects)
Metabolism & Metabolomics
Genomics and personalized strategies in nutrition
Nutrition during the early life cycle
Health issues and nutrition in the elderly
Phenotyping in clinical nutrition
Nutrition in acute and chronic diseases
The double burden of ''malnutrition'': Under-nutrition and Obesity
Prevention of Non Communicable Diseases (NCD)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


