Accessing elusive σ-type cyclopropenium cation equivalents through redox gold catalysis
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
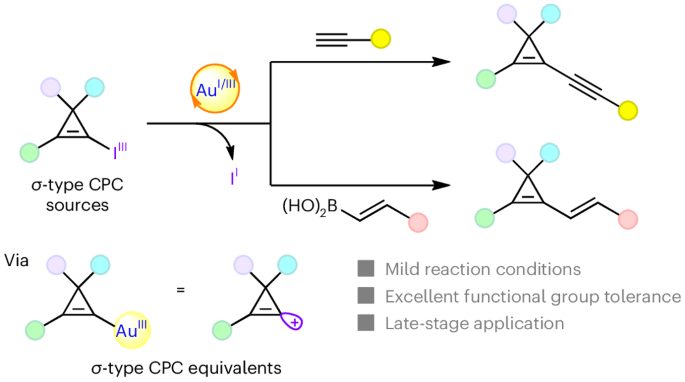
Cyclopropenes are the smallest unsaturated carbocycles. Removing one substituent from cyclopropenes leads to cyclopropenium cations (C3+ systems, CPCs). Stable aromatic π-type CPCs were discovered by Breslow in 1957 by removing a substituent on the aliphatic position. In contrast, σ-type CPCs—formally accessed by removing one substituent on the alkene—are unstable and relatively unexplored. Here we introduce electrophilic cyclopropenyl-gold(III) species as equivalents of σ-type CPCs, which can then react with terminal alkynes and vinylboronic acids. With catalyst loadings as low as 2 mol%, the synthesis of highly functionalized alkynyl- or alkenyl-cyclopropenes proceeded under mild conditions. A class of hypervalent iodine reagents—the cyclopropenyl benziodoxoles (CpBXs)—enabled the direct oxidation of gold(I) to gold(III) with concomitant transfer of a cyclopropenyl group. This protocol was general, tolerant to numerous functional groups and could be used for the late-stage modification of complex natural products, bioactive molecules and pharmaceuticals. The σ-type cyclopropenium cations (CPCs) are unstable species and currently underdeveloped. Now, an iodine(III)-based cyclopropenyl transfer reagent has been developed, which can generate electrophilic cyclopropenyl-gold(III) species as equivalents of σ-type CPCs. The synthetic potential has been demonstrated by the transfer of σ-type CPCs to terminal alkynes and vinylboronic acids.
通过氧化还原金催化获得难以捉摸的σ型环丙烯酸阳离子当量
环丙烯是最小的不饱和碳环。从环丙烯中去除一个取代基,就会产生环丙烯阳离子(C3+ 系统,CPCs)。1957 年,Breslow 通过去除脂肪族位置上的一个取代基,发现了稳定的芳香族 π 型 CPC。相比之下,通过移除烯上的一个取代基而形成的 σ 型 CPC 并不稳定,相对来说尚未得到研究。在这里,我们引入了亲电性环丙烯基金(III)物种作为 σ 型 CPC 的等价物,它们可以与末端炔烃和乙烯基硼酸反应。催化剂负载量低至 2 摩尔%时,就能在温和的条件下合成高度官能化的炔基或烯基环丙烯。一类高价碘试剂--环丙烯基苯碘硼烷(CpBXs)--可将金(I)直接氧化成金(III),同时转移环丙烯基。该方案具有通用性,对多种官能团具有耐受性,可用于复杂天然产物、生物活性分子和药物的后期改性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


